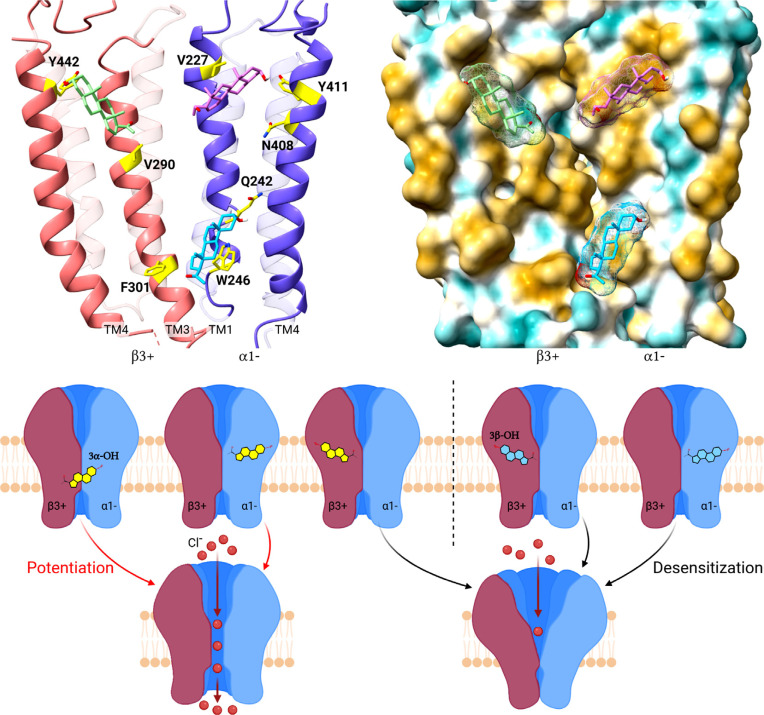
Fig. (2).
Models of neurosteroid binding sites on α1β3 GABAA receptors and the effects of their occupancy on channel gating. (Top left) Ribbon diagram of the interface between the β3(+) (salmon) and α1(-) (blue) subunits of a GABAA receptor (based on pdb 6HUO) with the neurosteroid 3α5α-P in its preferred docking poses in: an intra-β-subunit site between β3(V290) on TM3 and β3(Y442) on TM4; a β3(+)/α1(-) intersubunit site between α1(Q242), α1(W246) on TM1 and β3(F301) on TM3 and; an intra-α-subunit site between α1(V227) on TM1 and α1(Y411 and N408) on TM4. (Top right) Hydrophobic surface representation of 3α5α-P docked to the α1β3 GABAA receptor (brown most and turquoise least hydrophobic) illustrating that neurosteroids bind between the hydrophobic transmembrane α-helices on the receptor surface, interacting with both protein and annular lipid. (Lower panel) Cartoon illustrating that 3α5α-P (yellow steroid) occupancy of the β3(+)/α1(-) intersubunit site and/or the intra-α1-subunit site promotes channel activation. In contrast, occupancy of the intra-β3-subunit site by either 3α5α-P or 3β5α-P (blue steroid) and occupancy of the intra-α1-subunit site by 3β5α-P inhibits the receptor by promoting desensitization.