Abstract
Objective
The predictive factors for wheelchair dependence in patients with multiple system atrophy (MSA) are unclear. We aimed to explore the predictive factors for early-wheelchair dependence in patients with MSA focusing on clinical features and blood biomarkers.
Methods
This is a prospective cohort study. This study included patients diagnosed with MSA between January 2014 and December 2019. At the deadline of October 2021, patients met the diagnosis of probable MSA were included in the analysis. Random forest (RF) was used to establish a predictive model for early-wheelchair dependence. Accuracy, sensitivity, specificity, and area under the receiver operating characteristic curve (AUC) were used to evaluate the performance of the model.
Results
Altogether, 100 patients with MSA including 49 with wheelchair dependence and 51 without wheelchair dependence were enrolled in the RF model. Baseline plasma neurofilament light chain (NFL) levels were higher in patients with wheelchair dependence than in those without (P = 0.037). According to the Gini index, the five major predictive factors were disease duration, age of onset, Unified MSA Rating Scale (UMSARS)-II score, NFL, and UMSARS-I score, followed by C-reactive protein (CRP) levels, neutrophil-to-lymphocyte ratio (NLR), UMSARS-IV score, symptom onset, orthostatic hypotension, sex, urinary incontinence, and diagnosis subtype. The sensitivity, specificity, accuracy, and AUC of the RF model were 70.82 %, 74.55 %, 72.29 %, and 0.72, respectively.
Conclusion
Besides clinical features, baseline features including NFL, CRP, and NLR were potential predictive biomarkers of early-wheelchair dependence in MSA. These findings provide new insights into the trials regarding early intervention in MSA.
Keywords: Multiple system atrophy, Wheelchair dependence, Cohort study
Abbreviations: MSA, multiple system atrophy; UMSARS, unified multiple system atrophy rating scale; NLR, neutrophil-to-lymphocyte ratio; CRP, C-reactive protein; NFL, neurofilament light chain; RF, random forest; SCA, spinocerebellar ataxia; MSA-C, multiple system atrophy with predominate cerebellar ataxia; MSA-P, multiple system atrophy with predominate parkinsonism; OH, orthostatic hypotension; AUC, area under the receiver operating characteristic curve; TNF, tumor necrosis factor
1. Introduction
Multiple system atrophy (MSA) is a sporadic, adult-onset, and rare neurodegenerative disorder, clinically characterized by parkinsonism, cerebellar ataxia, and autonomic dysfunction [1]. MSA has unclear etiology and develops gradually. No effective treatment is available for MSA.
Requirement of walking aids, confinement to a wheelchair, and bedridden state are important clinical stages of MSA [2]. When MSA patients are confined to a wheelchair, many daily activities such as dressing, getting on and off the bed, bathing, and going to the toilet cannot be performed independently, thus leading to disability. The quality of life of patients with MSA gradually declines, leading to increased dependency and economic burden on their families. The mean duration of confinement to a wheelchair varied from 3.5 to 7 years in different studies [2], [3], [4], [5], [6], [7]. Several risk factors for wheelchair confinement in patients with MSA have been proposed. These include higher age of onset, higher Unified MSA Rating Scale (UMSARS) score, and early development of autonomic dysfunction [2], [3], [6]. However, discussions regarding the potential risk factors related to wheelchair confinement have mainly focused on clinical features. Additionally, data related to Chinese patients with MSA remain largely unknown.
Systemic inflammation may contribute to the progression of neurodegenerative diseases [8]. Previous studies have demonstrated that inflammation plays an important role in MSA [9], [10], [11].The blood neutrophil-to-lymphocyte ratio (NLR) and C-reactive protein (CRP) levels are easily available biomarkers indicating peripheral inflammation. We found that high NLR predicted short survival in MSA [12]. In addition, our previous study demonstrated that plasma neurofilament light chain (NFL) is a reliable biomarker for monitoring the progression of MSA [13]. We hypothesized that these blood biomarkers would predict wheelchair dependence in patients with MSA.
Random forest (RF) [14] is a widely used machine learning algorithm in healthcare [15], [16]. RF has exhibited important advantages over other methodologies, performed well in the classification task, and provided an important value to each feature [17]. However, RF has been rarely applied in the prediction of wheelchair dependence in patients with MSA.
Thus, the purpose of the present study was to establish a predictive model of early-wheelchair dependence in early-stage MSA based on the RF, focusing on clinical features and blood biomarkers.
2. Methods
We recruited participants from a prospective cohort of patients with MSA (disease duration <3 years) at the Department of Neurology, West China Hospital of Sichuan University between January 2014 and December 2019. All patients met the probable MSA based on the second consensus MSA criteria [1]. Patients underwent brain magnetic resonance imaging to exclude other neurological disorders and were screened for spinocerebellar ataxia (SCA) genes including SCA1, 2, 3, 6, and 7 to exclude the common forms of SCA. The exclusion criteria at baseline were as follows: 1) patients with a disease duration of more than 3 years, 2) patients confined to a wheelchair, 3) patients with incomplete blood indices, and 4) patients with urinary tract infections, bronchopneumonia, or aspiration pneumonia. All patients were followed up once a year via telephone or face-to-face interviews by neurologists.
MSA with predominantly cerebellar ataxia features was designated as MSA-C and that with predominantly parkinsonian features was designated as MSA-P [1]. All patients were independently interviewed and examined by a neurologist. Clinical data including age, sex, age of onset, disease duration, and symptom onset were collected. Disease duration was defined as the time from the date of disease onset to the evaluation date. Onset of symptoms was defined as the initial presentation of any motor symptoms (such as parkinsonism or cerebellar ataxia) or autonomic features except erectile dysfunction [1]. Disease severity was evaluated using the UMSARS, which was rated using a four-point scale [18]. Orthostatic hypotension (OH) was defined as a decrease in the systolic blood pressure by at least 30 mmHg or a decrease in the diastolic blood pressure by at least 15 mmHg after 3 min of standing following a previous 10-min interval in the recumbent position.
Baseline blood samples were obtained after overnight fasting. NFL, CRP, and NLR were analyzed in this study. Plasma NFL levels were quantified using the ultrasensitive Simoa technology (Quanterix, Billerica, MA, USA) on the automated Simoa HD-X platform according to the manufacturer’s instructions. Plasma samples with 1:4 dilution were used for the measurements. Calibrators, quality controls, and samples were measured in duplicates. The operators were blinded to the participants’ disease status. NLR was calculated by dividing the absolute number of neutrophils by the absolute number of lymphocytes obtained from the complete blood count.
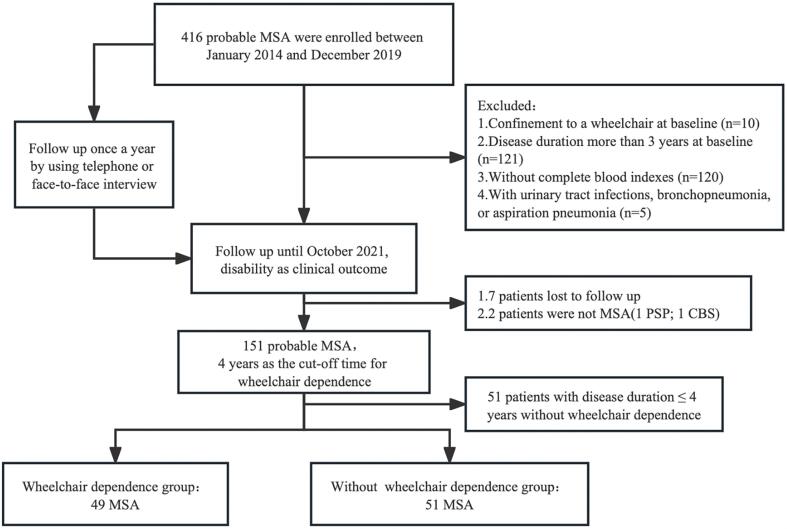
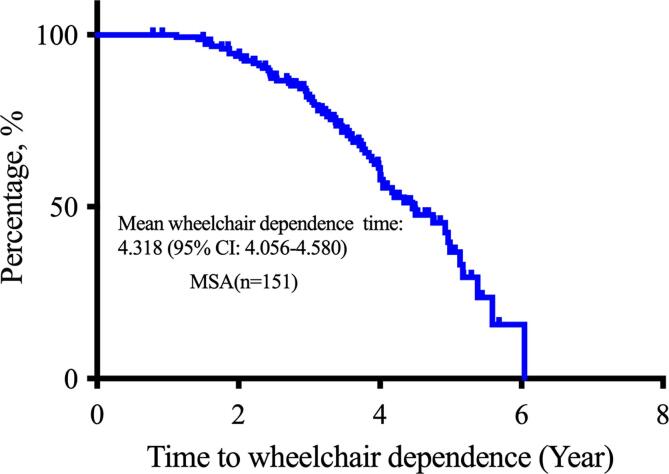
Wheelchair dependence was defined as permanent confinement to a wheelchair. Duration of wheelchair dependence was defined as the interval from the date of symptom onset to the date of confinement to a wheelchair. At the deadline of October 2021, 151 patients diagnosed with probable MSA were included in the study (Fig. 1). The mean duration from symptom onset to wheelchair dependence in 151 patients with MSA was 4.318 years (95 % confidence interval: 4.056–4.580) according to the Kaplan–Meier curve (Fig. 2). Therefore, the cut-off duration for early-wheelchair dependence in the present study was set to 4 years. According to the aim of the study, 51 patients with disease duration ≤4 years who were not wheelchair dependence at the time of set deadline, were excluded from the final analysis, since we did not know when the patients would develop wheelchair dependence. Thus, 100 patients were included in the final analysis. Based on the cut-off duration of early-wheelchair dependence of 4 years, patients were divided into two groups: 1) patients with wheelchair dependence (patients confined to a wheelchair at disease duration ≤4 years) and 2) patients without wheelchair dependence (patients confined or not confined to a wheelchair at disease duration >4 years).
Fig. 1.
Study flow diagram.
Fig. 2.
Kaplan–Meier curve of the duration from symptom onset to wheelchair dependence in 151 patients with multiple system atrophy.
2.1. Statistical analysis
Student’s t-test was used to compare the continuous variables, followed by the chi-squared test to compare the categorical variables between the groups. The following variables were included in the RF model: sex (female: 0, male: 1), age of onset, disease duration, diagnostic subtype (MSA-C: 0, MSA-P: 1), onset of symptoms (autonomic onset: 0, motor onset: 1, UMSARS-I score, UMSARS-II score, UMSARS-IV score, OH, urinary incontinence, and NFL, CRP, and NLR values. The RF algorithm randomly divided the data into two groups: 1) training set that included 80 % of the sample, and 2) test set that included the remaining 20 % of the sample. The training set was used for the construction of the early-wheelchair dependence model of MSA and the test set was used for internal validation of the model. Fivefold cross-validation was also performed. We used accuracy, sensitivity, specificity, and area under the receiver operating characteristic curve (AUC) to evaluate the performance of the RF model. The predictive power of variables in the RF model was measured using the mean decrease of the Gini index [19]. Gini index is a powerful measure of the randomness in the values of a dataset, which aims to decrease the impurities from the root nodes (at the top of decision tree) to the leaf nodes (vertical branches down the decision tree) of a decision tree model. The variables with the higher mean decrease of the Gini index are considered the most important. IBM SPSS Statistics (version 26.0; IBM Corp., Armonk, NY, USA) and R (version 4.0.2; The R foundation, Vienna, Austria) were used for statistical analyses. Statistical significance was set at p < 0.05.
3. Ethics statement
Approval was obtained from the Ethics Committee of West China Hospital of Sichuan University. Informed consent was obtained from all participates.
4. Results
Altogether, 100 patients with MSA (49 with wheelchair dependence and 51 without wheelchair dependence) were enrolled in the present study. The average follow-up period was more than 4 years (rang 2–7 years). The baseline demographic and clinical information of patients with MSA is presented in Table 1. The mean age of onset and mean disease duration of all patients with MSA at baseline were 57.82 ± 7.83 years and 1.90 ± 0.71 years, respectively. Patients with wheelchair dependence at baseline had a shorter disease duration, higher proportion of motor onset, and higher baseline UMSARS scores (UMSARS-I, UMSARS-II, and UMSARS-IV) than those without wheelchair dependence (P < 0.05). No significant differences were observed in other clinical features at baseline including diagnostic subtype, sex, age of onset, incidence of OH, and urinary incontinence between patients with and without wheelchair dependence.
Table 1.
Demographic and clinical information for MSA with and without wheelchair dependence at baseline.
| Variables | Total | With wheelchair dependence | Without wheelchair dependence | P-value |
|---|---|---|---|---|
| Number | 100 | 49 | 51 | – |
| Diagnosis subtype | ||||
| MSA-P | 40 | 19(38.8 %) | 21(41.2 %) | 0.806 |
| MSA-C | 60 | 30(61.2 %) | 30(58.8 %) | |
| Sex | ||||
| Male | 44 (44.0 %) | 23 (46.9 %) | 21 (41.2 %) | 0.562 |
| Female | 56 (56.0 %) | 26 (53.1 %) | 30 (58.8 %) | |
| Motor onset (%) | 60 (60.0 %) | 35 (71.4 %) | 25 (49.0 %) | 0.022* |
| Age of onset | 57.82 ± 7.83 | 59.01 ± 6.94 | 56.68 ± 8.51 | 0.139 |
| Disease duration | 1.90 ± 0.71 | 1.69 ± 0.65 | 2.10 ± 0.72 | 0.003* |
| UMSARS-I | 16.13 ± 6.54 | 18.18 ± 6.72 | 14.16 ± 5.78 | 0.002* |
| UMSARS-II | 18.32 ± 7.92 | 20.69 ± 8.77 | 16.04 ± 6.29 | 0.003* |
| UMSARS-IV | 2.14 ± 0.90 | 2.37 ± 0.76 | 1.92 ± 0.98 | 0.012* |
| OH (%) | 43 (43.0 %) | 24 (49.0 %) | 19 (37.3 %) | 0.236 |
| Urinary incontinence (%) | 67 (67.0 %) | 30 (61.2 %) | 37 (72.5 %) | 0.229 |
| NFL (pg/ml) | 40.47 ± 23.24 | 45.41 ± 27.89 | 35.73 ± 16.60 | 0.037* |
| CRP (mg/L) | 2.02 ± 2.42 | 2.31 ± 2.72 | 1.73 ± 2.07 | 0.234 |
| NLR | 2.21 ± 1.15 | 2.36 ± 1.45 | 2.07 ± 0.77 | 0.212 |
MSA: multiple system atrophy; MSA-P: multiple system atrophy with predominate parkinsonism; MSA-C: multiple system atrophy with predominate cerebellar ataxia; UMSARS: unified multiple system atrophy rating scale; OH: orthostatic hypotension; NFL: Neurofilament light chain; CRP: C-reactive protein; NLR: neutrophil-to-lymphocyte ratio.
* Significant difference.
Baseline plasma NFL levels of patients with wheelchair dependence were significantly higher than those of patients without wheelchair dependence (45.41 ± 27.89 vs 35.73 ± 16.60 pg/ml, P = 0.037). Although baseline CRP levels and NLR were higher in patients with wheelchair dependence, the difference was not statistically significant.
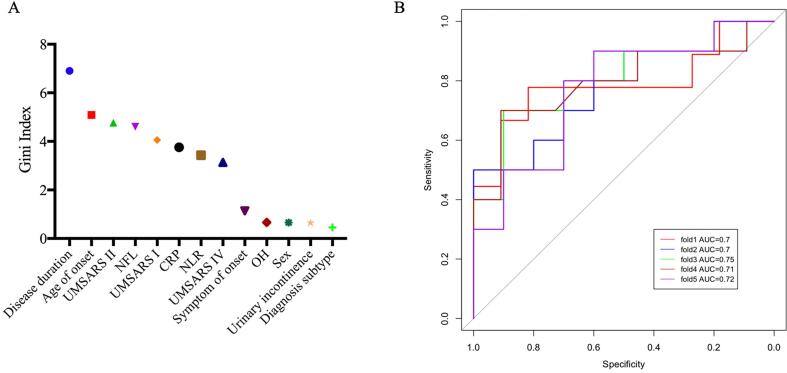
The Gini index depicted the predictive power of the variables that were included in the RF model (Fig. 3A). The five major predictive factors were disease duration, age of onset, UMSARS-II score, NFL, and UMSARS-I score; followed by CRP, NLR, UMSARS-IV score, symptom of onset, OH, sex, urinary incontinence, and diagnostic subtype. The fivefold cross-validation of the RF model showed that the sensitivity, specificity, and accuracy were 70.82 %, 74.55 %, and 72.29 %, respectively (Table 2). The receiver operating characteristic curve for the model is depicted in Fig. 3B. The mean AUC was 0.72.
Fig. 3.
The Gini index (A) and area under the receiver operating characteristic curve (B) of the random forest model.
Table 2.
Performances of the random forest model.
| Sensitivity (%) | Specificity (%) | Accuracy (%) | AUC | |
|---|---|---|---|---|
| Fold 1 | 63.64 | 77.78 | 70.00 | 0.70 |
| Fold 2 | 75.00 | 66.67 | 70.00 | 0.70 |
| Fold 3 | 72.73 | 77.78 | 75.00 | 0.75 |
| Fold 4 | 70.00 | 72.73 | 71.43 | 0.71 |
| Fold 5 | 72.73 | 77.78 | 75.00 | 0.72 |
| Mean | 70.82 | 74.55 | 72.29 | 0.72 |
AUC: area under receiver operating characteristic curve.
5. Discussion
To the best of our knowledge, the novelty of our study is that we conducted a prospective cohort study of patients with MSA, combining early clinical features and blood biomarkers to predict early-wheelchair dependence using a machine learning algorithm. We highlighted five major predictive factors for early-wheelchair dependence: disease duration, age of onset, UMSARS-II score, NFL, and UMSARS-I score. The sensitivity, specificity, accuracy, and AUC of the RF model for predicting early-wheelchair dependence were 70.82 %, 74.55 %, 72.29 %, and 0.72, respectively.
A Japanese study analyzed 230 patients with MSA and found that the mean duration from symptom onset to confinement to a wheelchair was 5 years [2]. Recently, a British study reported that the mean duration from symptom onset to wheelchair dependence was 5.4 years in 160 patients with pathologically confirmed MSA [7]. In the present study, the mean duration from symptom onset to wheelchair dependence was 4.3 years in 151 patients with MSA, which was similar to the results of previous studies [2], [7]. However, it was lower than that reported by another British study (6.7 years) with a small sample size of 83 patients with MSA [4]. Due to the short duration from symptom onset to wheelchair dependence, it is highly important to establish an early predictive model for wheelchair dependence using potential biomarkers.
Initially, we established a wheelchair dependence predictive model based on RF, combining clinical features and blood biomarkers. Disease duration and disease severity (UMSARS-II and UMSARS-I scores) were important predictive factors for wheelchair dependence in patients with MSA. In addition, we observed that these patients had a shorter disease duration and higher UMSARS-I and UMSARS-II scores at baseline. Therefore, our findings suggest that faster disease progression in the early stages of the disease can predict early occurrence of wheelchair dependence in patients with MSA.
Age of onset was also an important predictive factor for wheelchair dependence. Patients with wheelchair dependence had a relatively higher age of onset. Thus, our findings indicated that patients with MSA having a higher age of onset were more likely to develop wheelchair dependence within 4 years. This finding was consistent with that reported in a previous study [2].
NFL is essential for radial growth and structural stability of myelinated axons [20], which are released into the cerebrospinal fluid and blood during axonal damage [21]. Our previous study and other studies found that plasma NFL levels were higher in patients with MSA than in healthy controls [13], [22], [23], [24]. In addition, we found that NFL levels in these patients increased with disease progression and this increase could predict motor progression of MSA [13]. In the present study, we used a RF model to demonstrate that NFL is a valuable predictor of wheelchair dependence in MSA. These findings suggest that axonal injury may play an important role in the pathological mechanism of MSA. Our study highlights that NFL is a good quantitative biomarker for monitoring disease progression in future clinical trials.
Our RF model indicated that the role of CRP and NLR in predicting wheelchair dependence in MSA cannot be ignored. CRP and NLR are blood biomarkers that represent peripheral inflammation. Reportedly, systemic inflammation is related to the progression of neurodegenerative diseases [8]. Previous studies have demonstrated a correlation between inflammation and MSA [9], [10], [11], suggesting that inflammation plays an important role in the pathogenesis of MSA. There is a paucity of studies on peripheral inflammatory biomarkers in MSA. It has been reported that NLR is a predictive factor for the prognosis of cardiovascular diseases [25], cerebrovascular disease [26], and amyotrophic lateral sclerosis [27]. Our previous study reported that high NLR predicted short survival in MSA [12]. Patients with wheelchair dependence had relatively higher CRP levels and NLR at baseline. We hypothesized that patients with early wheelchair dependence would have a more severe inflammatory reaction in the early stage of MSA. Our study showed that both CRP level and NLR were predictive biomarkers for wheelchair dependence in MSA, which further supported the important role of peripheral inflammation in the prognosis of MSA.
A major strength of our study is that we conducted a prospective cohort study of patients with MSA, combining clinical features and blood biomarkers to predict wheelchair dependence using a machine learning algorithm. We highlighted five major predictive factors for wheelchair dependence: disease duration, age of onset, UMSARS-II score, NFL, and UMSARS-I score. These findings provide new insights into the trials regarding early intervention in MSA.
The present study has some limitations. First, we enrolled patients with MSA who had a disease duration of <3 years and underwent NFL measurement at baseline. Therefore, the sample size of MSA patients in our study was relatively small, which might have influenced the performance of the RF model. Second, peripheral inflammatory biomarkers were not comprehensive. Inflammatory biomarkers such as IL-1, IL-2, IL-6, IL-10 and tumor necrosis factor (TNF)-α could be used to improve the predictive model in the future. Third, all patients were clinically diagnosed without a postmortem diagnosis. However, we confirmed the diagnosis of probable MSA through regular annual follow-up.
6. Conclusion
This is the first study to construct a predictive model for early-wheelchair dependence combining clinical features and blood biomarkers and using a machine learning algorithm in a prospective MSA cohort. The sensitivity, specificity, accuracy, and AUC of the RF model for predicting early-wheelchair dependence were 70.82 %, 74.55 %, 72.29 %, and 0.72, respectively. Besides clinical features, baseline features including NFL, CRP, and NLR were potential predictive biomarkers of early-wheelchair dependence in MSA.
Funding
The present study was supported by the funding of 1.3.5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (Grant No. 2022HXFH023), Sichuan Science and Technology Program (Grant No. 2022ZDZX0023), and Sichuan Postdoctoral Program (Grant No. TB2022043).
CRediT authorship contribution statement
Lingyu Zhang: Conceptualization, Methodology, Software, Formal analysis, Writing – original draft, Writing – review & editing. Yanbing Hou: Conceptualization, Methodology, Software, Formal analysis, Writing – original draft, Writing – review & editing. Xiaojing Gu: Resources. Bei Cao: Resources. Qianqian Wei: Resources. Ruwei Ou: Resources. Kuncheng Liu: Resources. Junyu Lin: Resources. Tianmi Yang: Resources. Yi Xiao: Resources. Bi Zhao: Resources. Huifang Shang: Conceptualization, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank all subjects for their participation in the study.
References
- 1.Gilman S., Wenning G.K., Low P.A., Brooks D.J., Mathias C.J., Trojanowski J.Q., Wood N.W., Colosimo C., Durr A., Fowler C.J., Kaufmann H., Klockgether T., Lees A., Poewe W., Quinn N., Revesz T., Robertson D., Sandroni P., Seppi K., Vidailhet M. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe H., Saito Y., Terao S., Ando T., Kachi T., Mukai E., Aiba I., Abe Y., Tamakoshi A., Doyu M., Hirayama M., Sobue G. Progression and prognosis in multiple system atrophy: an analysis of 230 Japanese patients. Brain J. Neurol. 2002;125(5):1070–1083. doi: 10.1093/brain/awf117. [DOI] [PubMed] [Google Scholar]
- 3.Tada M., Onodera O., Tada M., Ozawa T., Piao Y.-S., Kakita A., Takahashi H., Nishizawa M. Early development of autonomic dysfunction may predict poor prognosis in patients with multiple system atrophy. Arch. Neurol. 2007;64(2):256. doi: 10.1001/archneur.64.2.256. [DOI] [PubMed] [Google Scholar]
- 4.O'Sullivan S.S., Massey L.A., Williams D.R., Silveira-Moriyama L., Kempster P.A., Holton J.L., Revesz T., Lees A.J. Clinical outcomes of progressive supranuclear palsy and multiple system atrophy. Brain J. Neurol. 2008;131(5):1362–1372. doi: 10.1093/brain/awn065. [DOI] [PubMed] [Google Scholar]
- 5.Starhof C., Korbo L., Lassen C.F., Winge K., Friis S. Clinical features in a danish population-based cohort of probable multiple system atrophy patients. Neuroepidemiology. 2016;46(4):261–267. doi: 10.1159/000444325. [DOI] [PubMed] [Google Scholar]
- 6.Lieto M., Roca A., Bruzzese D., Antenora A., Alfieri G., Saccà F., Bellofatto M., Bilo L., Barbato S., De Michele G., Filla A. Longitudinal study of a cohort of MSA-C patients in South Italy: survival and clinical features. Neurol. Sci. 2019;40(10):2105–2109. doi: 10.1007/s10072-019-03948-7. [DOI] [PubMed] [Google Scholar]
- 7.Miki Y., Foti S.C., Asi Y.T., Tsushima E., Quinn N., Ling H., et al. Improving diagnostic accuracy of multiple system atrophy: a clinicopathological study. Brain J. Neurol. 2019;142(9):2813–2827. doi: 10.1093/brain/awz189. [DOI] [PubMed] [Google Scholar]
- 8.Perry V.H., Nicoll J.A., Holmes C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 9.Zhou X., Wang C., Chen Z., Peng Y., Peng H., Hou X., Ye W., Qiu R., Xia K., Tang B., Jiang H. Association of TNF-α rs1799964 and IL-1β rs16944 polymorphisms with multiple system atrophy in Chinese Han population. Int. J. Neurosci. 2018;128(8):761–764. doi: 10.1080/00207454.2017.1418346. [DOI] [PubMed] [Google Scholar]
- 10.Engen P.A., Dodiya H.B., Naqib A., Forsyth C.B., Green S.J., Voigt R.M., Kordower J.H., Mutlu E.A., Shannon K.M., Keshavarzian A. The potential role of gut-derived inflammation in multiple system atrophy. J. Parkinsons Dis. 2017;7(2):331–346. doi: 10.3233/JPD-160991. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman E., Hall S., Surova Y., Widner H., Hansson O., Lindqvist D., Duda J. Proinflammatory cytokines are elevated in serum of patients with multiple system atrophy. PLoS One. 2013;8(4):e62354. doi: 10.1371/journal.pone.0062354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L., Cao B., Hou Y., Wei Q., Ou R., Zhao B., et al. High neutrophil-to-lymphocyte ratio predicts short survival in multiple system atrophy. npj Parkinson's Dis. 2022;8:1:11. doi: 10.1038/s41531-021-00267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L., Cao B., Hou Y., Gu X., Wei Q., Ou R., Zhao B., Luo C., Shang H. Neurofilament light chain predicts disease severity and progression in multiple system atrophy. Movement Disorders. 2022;37(2):421–426. doi: 10.1002/mds.28847. [DOI] [PubMed] [Google Scholar]
- 14.Breiman L. Random forests. Mach. Learn. 2001;45(1):5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 15.Dwyer D.B., Falkai P., Koutsouleris N. Machine learning approaches for clinical psychology and psychiatry. Annu. Rev. Clin. Psychol. 2018;14:91–118. doi: 10.1146/annurev-clinpsy-032816-045037. [DOI] [PubMed] [Google Scholar]
- 16.Sarica A., Cerasa A., Quattrone A. RandoM FOREST ALGORITHM FOR THE CLASSIFICATION OF NEUROIMAGING DATA IN Alzheimer's disease: A systematic review. Front. Aging Neurosci. 2017;9:329. doi: 10.3389/fnagi.2017.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caruana R, Niculescu-Mizil A: An empirical comparison of supervised learning algorithms. In: Proceedings of the 23rd international conference on Machine learning. 2006: 161-8.
- 18.Wenning G.K., Tison F., Seppi K., Sampaio C., Diem A., Yekhlef F., Ghorayeb I., Ory F., Galitzky M., Scaravilli T., Bozi M., Colosimo C., Gilman S., Shults C.W., Quinn N.P., Rascol O., Poewe W. Development and validation of the Unified Multiple System Atrophy Rating Scale (UMSARS) Movement Disorders. 2004;19(12):1391–1402. doi: 10.1002/mds.20255. [DOI] [PubMed] [Google Scholar]
- 19.Strobl C., Boulesteix A.-L., Augustin T. Unbiased split selection for classification trees based on the Gini index. Comput. Stat. Data Anal. 2007;52(1):483–501. [Google Scholar]
- 20.Yuan A., Rao M.V., Veeranna, Nixon R.A. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb. Perspect. Biol. 2017;9(4):a018309. doi: 10.1101/cshperspect.a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalil M., Teunissen C.E., Otto M., Piehl F., Sormani M.P., Gattringer T., Barro C., Kappos L., Comabella M., Fazekas F., Petzold A., Blennow K., Zetterberg H., Kuhle J. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018;14(10):577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 22.Wang S.-Y., Chen W., Xu W., Li J.-Q., Hou X.-H., Ou Y.-N., Yu J.-T., Tan L., Zhu L.-Q. Neurofilament light chain in cerebrospinal fluid and blood as a biomarker for neurodegenerative diseases: A systematic review and meta-analysis. J. Alzheimer's Dis.: JAD. 2019;72(4):1353–1361. doi: 10.3233/JAD-190615. [DOI] [PubMed] [Google Scholar]
- 23.Marques T.M., van Rumund A., Oeckl P., Kuiperij H.B., Esselink R.A.J., Bloem B.R., Otto M., Verbeek M.M. Serum NFL discriminates Parkinson disease from atypical parkinsonisms. Neurology. 2019;92(13):e1479–e1486. doi: 10.1212/WNL.0000000000007179. [DOI] [PubMed] [Google Scholar]
- 24.Hansson O., Janelidze S., Hall S., Magdalinou N., Lees A.J., Andreasson U., Norgren N., Linder J., Forsgren L., Constantinescu R., Zetterberg H., Blennow K. Blood-based NfL: A biomarker for differential diagnosis of parkinsonian disorder. Neurology. 2017;88(10):930–937. doi: 10.1212/WNL.0000000000003680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dentali F., Nigro O., Squizzato A., Gianni M., Zuretti F., Grandi A.M., Guasti L. Impact of neutrophils to lymphocytes ratio on major clinical outcomes in patients with acute coronary syndromes: A systematic review and meta-analysis of the literature. Int. J. Cardiol. 2018;266:31–37. doi: 10.1016/j.ijcard.2018.02.116. [DOI] [PubMed] [Google Scholar]
- 26.Tokgoz S., Keskin S., Kayrak M., Seyithanoglu A., Ogmegul A. Is neutrophil/lymphocyte ratio predict to short-term mortality in acute cerebral infarct independently from infarct volume? J. Stroke Cerebrovasc. Dis. 2014;23(8):2163–2168. doi: 10.1016/j.jstrokecerebrovasdis.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Choi S.J., Hong Y.H., Kim S.M., Shin J.Y., Suh Y.J., Sung J.J. High neutrophil-to-lymphocyte ratio predicts short survival duration in amyotrophic lateral sclerosis. Sci. Rep. 2020;10:1:428. doi: 10.1038/s41598-019-57366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]