Figure 5. Tumorigenicity and phenoptypic assessment in xenograft assays.
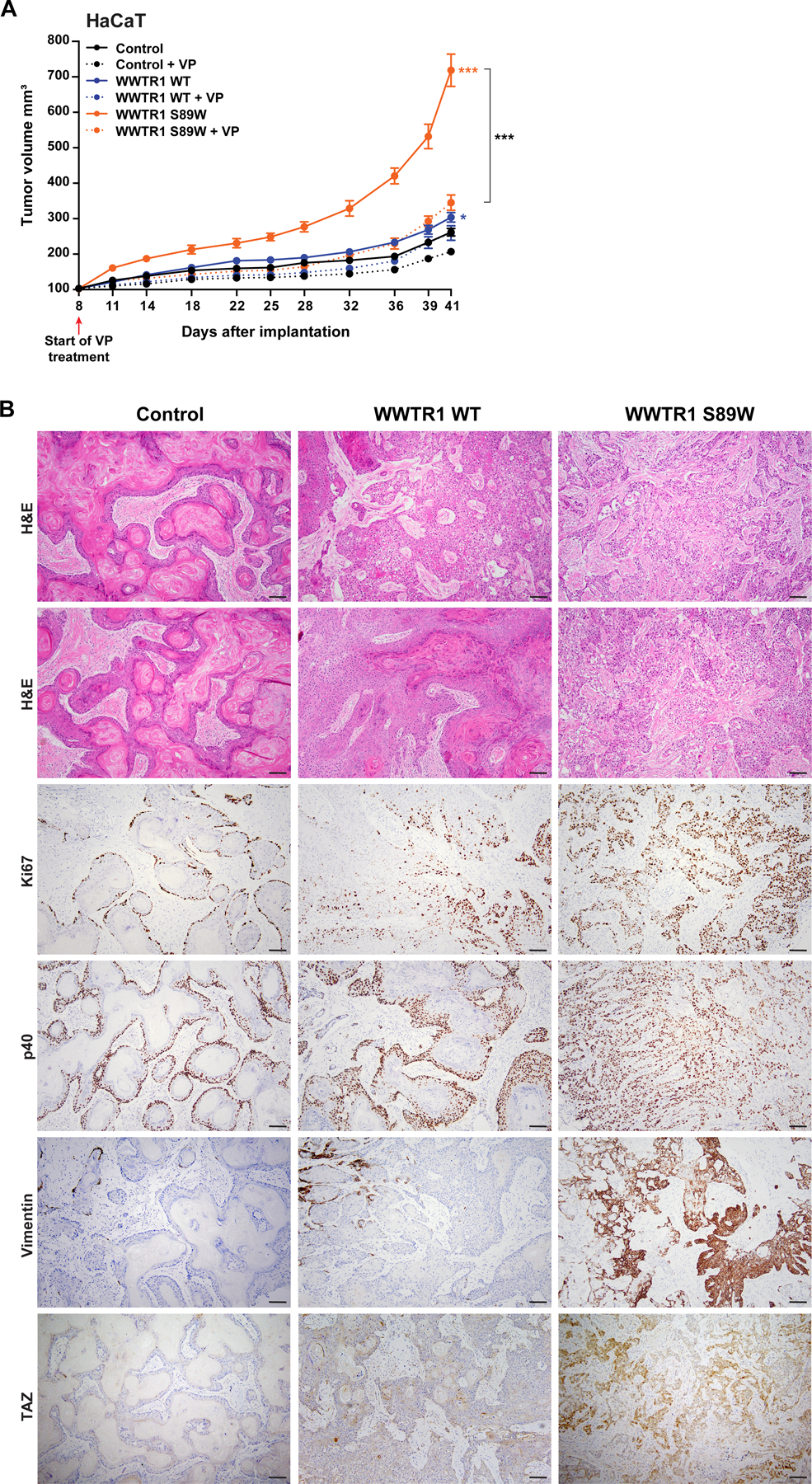
(A) Tumor growth of xenografts derived from immortalized human keratinocyte HaCaT cells expressing empty vector (Control), WWTR1 wild-type (WWTR1 WT) and WWTR1 S89W treated with 2 µM Verteporfin (VP) or DMSO as control. VP, Verteporfin. Experiments were performed in 5 mice per condition. Mean ±SEM; n.s., not significant; *P < .05, **P < .01, ***P < .001; two‐tailed unpaired t-test. (B) Representative micrographs of sections of xenograft tumors derived from HaCaT cells expressing Control, WWTR1 WT and WWTR1 S89W subjected to hematoxylin‐and‐eosin (H&E) staining, Ki67, p40, Vimentin and TAZ immunohistochemical analysis (scale bar, 100 µm).
