Abstract
The discovery and development of Caenorhabditis elegans as a model organism was influential in biology, particularly in the field of aging. Many historical and contemporary studies have identified thousands of lifespan-altering paradigms including genetic mutations, transgenic gene expression, and hormesis, a beneficial, low-grade exposure to stress. With its many advantages, including a short lifespan, easy and low-cost maintenance, fully sequenced genome sequence with homology to almost two-third of all human genes, C. elegans has quickly been adopted as an outstanding model for stress and aging biology. Here, several standardized methods are surveyed for measuring lifespan and healthspan that can be easily adapted into almost any research environment, especially those with limited equipment and funds. The incredible utility of C. elegans are featured, highlighting the capacity to perform powerful genetic analyses in aging biology without the necessity of extensive infrastructure. Finally, limitations of each analysis and alternative approaches are discussed for consideration.
Keywords: C. elegans, stress, aging, unfolded protein response, endoplasmic reticulum, mitochondria, heat-shock response
SHORT ABSTRACT:
C. elegans serve as an excellent model system with robust and low-cost methods for surveying healthspan, lifespan, and resilience to stress.
INTRODUCTION:
Since the time of the publication of ‘The genetics of Caenorhabditis elegans’, one of the most influential articles by Sydney Brenner in 1974, this microscopic worm has been considered as an outstanding model system to study biological mysteries1. In 1977 Michael R. Klass published the method for measuring the lifespan of C. elegans and established this model system to study aging2. The investigation to understand the relationship between stress and longevity has been started with the identification of a single mutation in the age-1 gene, which resulted in a lifespan extension in C. elegans3. Furthermore, contemporary studies have identified other lifespan-increasing mutations, which revealed long-lived mutant worms that exhibit increased resistance to stress4–6. With its many advantages including a short lifespan, easy maintenance, completely sequenced genome containing homology to about two-third of all human disease-causing genes, availability and ease of using RNA interference (RNAi) libraries, and physiological similarity with humans7–9, C. elegans has quickly been adopted as an outstanding model for stress and aging biology.
Perhaps the greatest utilities of C. elegans are its extremely low cost of maintenance, ease of experimentation, and the variety of genetic tools available for studies. C. elegans are typically grown on solid agar medium with an E. coli food source. Two commonly used E. coli strains are standard OP50, a B strain and perhaps the most commonly used10, and HT115, a K-12 strain that is used primarily for RNAi experiments11, 12. The HT115 K-12 strain carries a deletion in RNAIII RNase, a mutation which is essential for RNAi methods, where plasmids expressing dsRNA corresponding to individual C. elegans genes are used. The dsRNA feeding vectors allow for robust knockdown of C. elegans genes without the need for complex crosses or genome editing, as bacteria carrying these plasmids can be directly fed to nematodes. Thousands of these bacterial RNAi vectors exist in the HT115 background, including the most popular Vidal RNAi library with >19,000 individual RNAi constructs13 and the Ahringer RNAi library with 16,757 RNAi constructs14. However, the OP50 and HT115 bacterial diets have major differences in metabolic profile, including differences in Vitamin B1215, 16. Therefore, it is recommended to perform all experiments on a single bacteria source, if possible, to avoid gene-diet interactions that may introduce multiple confounding factors as previously described17–19. Due to the ease of maintaining animals on OP50, animals are maintained on OP50 for all the experimental conditions described here, but all experiments are performed on HT115 as previously described20. Briefly, animals are maintained at OP50 and transferred to HT115 post synchronization (after bleaching) for consistency between RNAi vs. non-RNAi experiments. Alternatively, an RNAi-competent OP50 strain carrying a similar deletion of RNAIII RNase found in the E. coli K12 HT115 strain can also be used21.
Perhaps one major limitation to RNAi experiments in C. elegans is the concern of knockdown efficiency. While knockdown efficiency can be validated via qPCR or western blotting, these require expensive equipment and reagents, and are limited to bulk analysis. This is even more of a concern looking at specific cells, such as neurons, which are refractory (less sensitive) to RNAi. While RNAi efficiency in specific cells can be enhanced via overexpression of SID-1, the transmembrane protein essential for dsRNA uptake22, this is still limited to the cell type-specific expression patterns of the promoters used for these constructs and thus gene knockouts and mutations are the most foolproof means of depleting gene functions. Beyond RNAi-mediated knockdown, C. elegans are also highly amenable to genome editing with CRISPR-based strategies23–25 and transgenic construct over-expression through microinjections, with the option to integrate transgenic constructs through irradiation or transposon based integration26–29. However, these methods require expensive microinjection equipment and the high cost of guide RNAs or Cas9 enzyme may prohibit these methods in institutions with limited funding. Instead, thousands of transgenic lines and mutants are readily available for a few dollars both at the Caenorhabditis Genetics Center (CGC) and National Bioresource Project (NBRP). The NBRP offers isolated mutants for a large number of C. elegans genes, including published and therefore verified mutant strains, mutants derived from pilot projects, and mutants that have yet to be characterized. In contrast, CGC is a depository of mostly published and established C. elegans lines from the research community. Both ship strains world-wide at very reasonable rates and offer a wide variety of options for those with limited capacity to synthesize strains in-house.
Here, a curated methods collection is offered, which are likely to be the lowest cost methods for assaying lifespan and healthspan in C. elegans. All the methods presented here require low-cost equipment and supplies, and only utilize strains readily available from the CGC. Perhaps most prohibitive for longevity and survival assays in C. elegans is the cost of Nematode Growth Media (NGM) plates. Since C. elegans are hermaphrodites and self-fertilize, standard survival assays require that adult animals be continuously moved away from their progeny to avoid contamination from offspring. Not only is this process time-consuming but can become expensive due to the necessity of approximately 100 plates per condition to run a single lifespan. Here, two alternatives are provided: utilization of the temperature-sensitive germline-less mutant, glp-4(bn2), or chemical sterilization using 5-fluoro-2’-deoxyuridine (FUDR). glp-4 encodes a valyl aminoacyl tRNA synthetase, and the temperature sensitive glp-4(bn2) are reproductively deficient at restrictive temperatures due to decreased protein translation30, 31. FUDR is a robust method to chemically sterilize C. elegans by preventing DNA replication, thus inhibiting reproduction32. Although FUDR can be prohibitively expensive for some labs, only a small amount is required to chemically sterilize worms and its stability in powder form may make it feasible for most groups. Utilizing the temperature sensitive glp-4(bn2) mutant is certainly the cheapest option, as the only requirement is an incubator to shift the animals to the restrictive 25°C; however, it should be noted that growth at 25°C may cause mild heat-stress33, 34. Regardless of the method, using sterile animals can significantly decrease the costs of consumables required for age-related assays.
To study aging, standard lifespan assays are conventional as paradigms that alter longevity have direct impacts on aging. However, measurements of healthspan and stress tolerance present additional information on the health of the organism. Here, several methods are offered to measure healthspan: 1) fecundity as a measure of reproductive health; 2) brood size as a measure of developmental health and viability of laid offspring; and 3) locomotory behavior as a measure of muscle function and motility, both of which are directly correlated with aging. Additionally, assays of stress tolerance are offered: survival to ER stress, mitochondrial/oxidative stress, and thermal stress survival. Indeed, animals with increased resistance to ER stress35, 36, mitochondrial stress37, and thermal stress38 exhibit increased lifespan. To apply ER stress, we expose C. elegans to tunicamycin, which blocks N-linked glycosylation and causes accumulation of misfolded proteins39. We induce mitochondrial/oxidative stress by exposure to paraquat, which induces superoxide formation specifically in the mitochondria40. Heat stress is applied through incubation of animals at 34–37°C33, 41. All the assays described here can be performed with minimal equipment and funds and offer a variety of tools to study aging to diverse groups.
PROTOCOL:
1. Growth and maintenance of C. elegans.
1.1. Pouring Nematode Growth Media (NGM) plates.
Grow C. elegans on standard 2% agar plates with Nematode Growth Media (NGM) consisting of 1 mM CaCl2, 5 μg/mL cholesterol, 25 mM KPO4 pH 6.0, 1 mM MgSO4, 0.25% w/v Bacto-Peptone, and 51.3 mM NaCl.
For 1L of NGM-agar plates, measure out 2.5 g of Bacto-Peptone, 3.0 g NaCl, and 20 g of agar into a 1 L flask with a stir bar. Note: we recommend standardizing a specific agar source, as we have seen variability in stiffness between brands, which may affect reproducibility. Here, we strictly use Bacto-Agar. Additionally, we recommend adding agar directly into the flask being autoclaved as agar will not dissolve completely without heating and transferring solution containing agar will result in loss of agar and errors in concentration.
Add dH2O up to 970 mL. Note: 30 mL of liquid additives post-sterilization will bring the final volume to 1 L. In our hands, ~951 mL of dH2O will be required to reach 970 mL final volume.
Sterilize NGM-agar solution using a standard autoclave or mediaclave for efficient sterilization. Note: at this point, sterile NGM-agar can be stored for several months at room temperature. If being stored, NGM-agar can be reliquefied in a microwave with 15–45 s pulses to prevent solution from boiling over, or in a heated water bath.
Let the solution stir until it is cooled to 60–75°C. Stirring while cooling is important to prevent uneven cooling, which may cause some agar to solidify.
While solution cools, heat a water bath or bead bath to 65–70°C.
Once the solution cools to 60–75°C, add liquid additives: 2.0 mL of 0.5 M CaCl2, 1 mL 5 mg/mL cholesterol, 25 mL 1 M KPO4 pH 6.0, and 0.5 M MgSO4 (see Table 1 for recipes for all reagents), and allow solution to mix for ~5 min to ensure complete mixing. Note: drugs can also be included in plates here (e.g., add 1 mL of 100 mg/mL carbenicillin and 1 mL of 1 M IPTG; add 10 mL of 2.5 mg/mL tunicamycin; add 10 mL of 400 mM paraquat).
Submerge flask containing NGM-agar in a 65–70°C water bath to prevent NGM-agar from solidifying while pouring plates.
Pipette 9–11 mL of solution into each 60 mm plate, or 20–30 mL of solution into each 100 mm plate. Note: We recommend using the smallest pipette volume available to avoid NGM leaks caused by air expanding in the pipette. Pipetting up 1–2mL more media than will be added to each plate to avoid completely emptying the pipette will help prevent bubble formation. Alternatively, plates can be hand-poured directly from the bottle into a plate, but we highly recommend pipetting to ensure plates with equal volume. Equal volume plates are important to ensure similar concentrations of solutions when using methods where solutions are applied directly on top of a plate (see step 17). Equal volumes also allow for easy microscopy to maintain similar focal plane across plates.
Place the pipette back in the heated solution to maintain the temperature and to prevent NGM-agar from solidifying.
Repeat steps 10–11 until all plates are poured.
Allow NGM-agar plates to solidify overnight.
After plates have solidified, store plates for up to 3 months at 4 °C or move onto step 14 for seeding plates with bacteria. Store plates in sealed containers to help retain moisture to maintain plate quality.
Grow a culture of OP50 in LB or equivalent media of choice for 24–48 hrs at ambient temperature (~22–25°C); or grow a culture of HT115 in LB + antibiotics (ampicillin/carb + tetracycline is recommended for HT115) shaking at 37°C for 12–16 hrs. Note: We recommend growing OP50 at room temperature because we have seen that growth at 37°C causes more aggressive growth of OP50, which affects C. elegans lifespan. In contrast, HT115 has a slower growth rate and makes less dense cultures, thus for HT115 we recommend growth at 37°C with shaking.
Seed a volume of ~100–200 μL of a saturated OP50/HT115 culture onto a 60 mm plate or 1 mL for a 100 mm plate.
Let plates dry overnight on benchtop and allow for an additional day to dry if plates are still wet. Store plates in sealed containers at 4 °C for ~2 months.
Optional: drugs can be added directly onto seeded NGM-agar plates: e.g., add 100 μL of 10 mg/mL FUDR solution to chemically sterilize worms.
Table 1.
Recipes for reagents and media for protocols.
| Reagent | Recipe |
|---|---|
| Bleach solution | 1.8% (v/v) sodium hypochlorite, 0.375 M KOH |
| Carbenicillin | 100 mg/mL stock solution (1000x) in water. Store at 4 °C for up to 6 months or −20 °C for long-term storage |
| FUDR | 10 mg/mL solution in water. Store at −20 °C. |
| IPTG | 1 M solution in water. |
| Lysogeny Broth (LB) | In this protocol, commercial LB was used (see materials), but all standard LB home-made recipes using Bacto-tryptone, yeast extract, and NaCl are sufficient. |
| M9 solution | 22 mM KH2PO4 monobasic, 42.3 mM Na2HPO4, 85.6 mM NaCl, 1 mM MgSO4 |
| Nematode Growth Media (NGM) | 1 mM CaCl2, 5 μg/mL cholesterol, 25 mM KPO4 pH 6.0, 1 mM MgSO4, 2% (w/v) agar, 0.25% (w/v) Bacto-Peptone, 51.3 mM NaCl |
| NGM RNAi plates | 1 mM CaCl2, 5 μg/mL cholesterol, 25 mM KPO4 pH 6.0, 1 mM MgSO4, 2% (w/v) agar, 0.25% (w/v) Bacto-Peptone, 51.3 mM NaCl, 1 mM IPTG, 100 μg/mL carbenicillin/ampicillin. Store at 4 ° C in dark for up to 3 months |
| NGM RNAi DMSO | 1 mM CaCl2, 5 μg/mL cholesterol, 25 mM KPO4 pH 6.0, 1 mM MgSO4, 2% (w/v) agar, 0.25% (w/v) Bacto-Peptone, 51.3 mM NaCl, 1 mM IPTG, |
| (control for tunicamycin) | |
| NGM RNAi TM | 1 mM CaCl2, 5 μg/mL cholesterol, 25 mM KPO4 pH 6.0, 1 mM MgSO4, 2% (w/v) agar, 0.25% (w/v) Bacto-Peptone, 51.3 mM NaCl, 1 mM IPTG, 100 μg/mL carbenicillin/ampicillin; 1% DMSO, 25 ng/μL tunicamycin |
| Paraquat | 400 mM solution in water – should be prepared fresh |
| Tetracycline | 10 mg/mL stock solution (500x) in 100% ethanol. Store at −20 °C |
| Tunicamycin | 2.5 mg/mL stock solution in 100% DMSO. Store at −80 °C for long-term storage. This is a 100x solution (25 ng/μL working solution) |
1.2. Maintaining C. elegans stocks.
Properly label the bottom of a seeded NGM-agar plate. Label the edges on the bottom of the plate to prevent obstructing passage of light on standard dissection microscopes.
Using a standard dissection microscope, scoop bacteria of choice onto C. elegans pick. Note: in this protocol, we utilize a pick consisting of a 90%/10% platinum/iridium wire attached to the end of a glass Pasteur pipette.
Using the bacteria, collect 10–20 eggs, L1, L2, or L3 animals and transfer onto newly labeled seeded NGM-agar plate. Note: it is best to collect younger animals; in our experience for standard wild-type animals, moving 10–20 eggs/young animals will allow the plate to grow at 15°C without starvation. For transgenic or mutant animals with decreased fecundity, more animals should be moved into the plate.
For animals with wild-type fecundity and grown at 15°C, repeat steps 1–3 very 7 days to maintain a weekly stock. For animals grown at 20°C, repeat steps 1–3 every 4–5 days to prevent starvation.
1.3. Synchronizing worms via bleaching.
A full, 60 mm NGM-agar plate (e.g., 1 week old stock plates grown at 15°C) will provide a sufficient number of animals for most standard assays described. Generally, one gravid adult (adults full of eggs) will provide 10–15 eggs42, and a full, 60 mm NGM-agar plate has anywhere from 100–200 gravid adults, providing ~1000–2000 eggs.
For larger scale experiments requiring more animals, cut a full 60 mm NGM-agar into 4–6 equal pieces and chunk onto seeded 100 mm plates for expansion. Chunking refers to cutting a piece of the NGM-agar plates containing worms and moving the entire chunk of agar+worms onto a new plate, worm-side down to allow the worms to crawl onto the new plate. Note: as a frame of reference, animals with wild-type fecundity will produce a full 100 mm plate if grown at 20 °C for 2–3 days after chunking.
To begin collecting the nematodes, pour a small amount of M9 onto plates containing worms taking care not to overfill the petri dish. Swirl the M9 gently to loosen worms off bacterial lawns.
Collect gravid adult worms with a serological pipette, taking care not to pierce the agar with the pipette tip. Note: Glass serological pipettes are recommended, as C. elegans tend to stick onto plastic. If glass pipettes are not available, it is recommended to start with a larger number of animals than needed, as some will be lost due to sticking to plastic pipettes.
Pellet the animals by centrifugation for 30 s at 1,100 × g. Aspirate out the supernatant. Note: the C. elegans pellet is a very loose pellet, so take care not to shake or disrupt the pellet while aspirating the supernatant.
While animals are centrifuging, prepare 5 mL of bleaching solution per strain (see Table 1 for recipe details): for 5 mL of solution, mix 1.5 mL of 6% sodium hypochlorite (bleach), 0.75 mL of 5 M NaOH or KOH, and 2.75 mL of dH2O. Safety: sodium hypochlorite and high concentration hydroxide solutions are corrosive, and thus it is recommended to wear gloves and a lab coat when handling.
Add 5 mL of bleaching solution to the worm pellet/M9 mixture.
Check the worms under a dissecting microscope every few mins until all the adult worm bodies have been dissolved and only eggs are left in the mix. Worm/bleach mix can be vigorously shaken to speed up the bleaching process. Note: leaving eggs inside a bleach mix for extended periods will result in damaging the eggs and will affect the viability of the animals. For wild-type animals, bleaching usually takes 4–6 mins with shaking. Thus, it is recommended to check the animals under a microscope in 30 s intervals starting from the 4 min mark.
Pellet the eggs by spinning down the egg/bleach mix for 30 s at 1,100 × g. Note: Some 15 mL conical tubes have gradient lines on the inside of the tube. For these tubes, we recommend spinning eggs at a higher speed (e.g., 30 s at 2,000 × g) to ensure that eggs pellet to the bottom of the tube and do not remain on gradient lines.
Aspirate out the bleaching solution. Note: an egg pellet is stiffer than a worm pellet, but can still be disrupted easily, so take care to not shake the tube after centrifuging.
Wash eggs by adding M9 up to 15 mL and inverting the tube 4–5 times to ensure that the eggs are fully dispersed in M9 solution.
Pellet eggs by centrifuging for 30 s at 1,100 × g and aspirate out M9 solution.
Repeat steps 11–12 for a total of 4 washes to eliminate any bleach from the egg mix.
Resuspend the eggs in 100uL to 2mL of M9 (depending on the total number of worms bleached) after the final wash. Shake eggs thoroughly to be break up clumps and ensure that pellet is fully resuspended. Note: alternatively, animals can be L1 arrested for a tighter temporal synchronization. For L1 arresting: add M9 to the egg pellet to ~10 mL in a 15 mL conical tube. Let the worms spin in a rotator for up to 24 hrs at 20 °C or at ambient temperature. L1 animals generally take 1/2 day less to reach adulthood compared to the timing of eggs described in step 16.
Approximate the egg concentration (or L1 concentration, see step 14 Note) by pipetting 4 μL of egg/M9 mixture onto an NGM-plate seeded with bacteria. Count and calculate how many eggs are present per μL plated. Repeat the count 3–4 times to improve the approximation. Approximating the egg concentration will ensure that enough animals are plated for appropriate sample size for experiments without overplating, which will cause starvation.
Based on the approximation, plate the appropriate number of eggs onto NGM-agar plates seeded with bacteria of choice. For OP50 plates, plate a maximum of 200 animals on a 60 mm plate and 1000 animals on a 100 mm plate. For HT115 plates, plate a maximum of 150 animals on a 60 mm plate and 600 animals on a 100 mm plate. Note: these are approximated numbers based on our lab conditions, and numbers may change based on thickness of your bacterial lawn. Eggs grown at 15 °C will take ~5 days to reach day 1 adulthood (~140 hrs to reach egg-laying maximal, gravid adult stage). Eggs grown at 20 °C will take ~4 days to reach day 1 adulthood (~96 hrs to reach egg-laying maximal, gravid adult stage). Eggs grown at 25 °C will take ~3.5 days to reach day 1 adulthood (~62 hrs to reach egg-laying maximal, gravid adult stage).
1.4. Egg-lay as an alternative method to synchronize C. elegans populations.
If bleaching protocols are not feasible (e.g., no centrifuge available), an alternative method to synchronize populations of C. elegans is to perform an egg-lay procedure. Keep in mind that this protocol is more labor-intensive and will result in smaller yields of animals.
For egg-laying, place 8–12 gravid adults onto a standard NGM-agar plate seeded with bacteria of choice and document the exact number of animals placed onto a plate. Note: egg-lay procedures should be performed at the temperature that will be used for experimentation.
Allow animals to lay eggs for 4–8 hrs. Note: duration animals are left on plate can be adjusted when needed. For example, a larger number of animals can be put on a plate for a shorter egg-lay duration when less time is available. C. elegans generally lay eggs in bursts, which can be estimated at a rate of ~5 eggs per hr for animals with wild-type fecundity43. Follow recommendations in section 1.3 step 16 to avoid overplating animals.
Remove all adult animals from the plate. Note: Any adult animals left on the plate will continue to lay eggs, resulting in an unsynchronized population.
Place eggs at 15 °C for ~5 days or 20 °C for ~4 days to reach day 1 adulthood.
2. Measuring longevity in C. elegans.
2.1. Standard lifespan.
Prepare NGM-agar plates by seeding plates with 100 μL bacteria of choice. For consistency, ensure that the same bacteria is used across all replicates. Since worms are moved every day during the egg-laying stages of adulthood, seed 5–7 sets of NGM-agar plates for the duration of the lifespan and 2–4 plates per strain to grow animals to adulthood (i.e., if using 8 plates of 15 animals for lifespans, you will need to seed 40–56 plates per condition).
Allow plates to dry overnight before storage. Note: we recommend that plates be stored at 4 °C and the required number of plates be removed from cold storage daily to prevent bacteria from making thick lawns that can make moving/counting lifespans difficult. Ensure that plates are warmed up prior to plating worms.
Collect a synchronized population of C. elegans using a standard bleaching assay as described in section 1.3–1.4.
Move 10–15 day 1 adult animals onto each of 8–12 plates. For a standard lifespan, it is recommended to start with ~120 animals to ensure that sample size does not drop too far below 100 after censorship events (e.g., 8 plates of 15 animals = 120 animals; 12 plates of 10 animals = 120 animals). Note: in our hands, 10–15 animals are a manageable number for most investigators, although 6 plates of 20 animals is also feasible to decrease the cost of consumables.
For the first 7–8 days or until progeny are no longer visible, adult animals should be moved away from their progeny every 1–2 days. Note: animals can be moved every other day to save materials, but care must be taken to ensure that eggs/larval animals are not transferred with the adult to prevent contamination of adult populations with progeny. In our hands, the simplest method is to move animals every day from days 1–3 when egg-laying is at its maximal, then switch to moving animals every other day for days 5–8 when egg-laying is minimal. With this method, it is not imperative to prevent transfer of eggs/larval animals during days 1–3 since the adults will be moved every day, and eggs/larvae cannot develop to adulthood in 1 day.
After animals have stopped producing progeny, lifespans are scored every other day until all animals have been scored as dead or censored. All dead or censored animals should be removed from the plate to avoid confusion and recounting the same animal. Note: death is scored as animals that exhibit no movement when gently touched with a pick. Censorship is scored as animals that are bagged, exhibit vulval/intestinal protrusions, or crawled up to the sides of the plate where they dessicate.
2.2. Lifespans with chemical sterilization using FUDR.
Prepare NGM-agar plates by seeding plates with 100 μL bacteria of choice. For consistency, ensure that the same bacteria is used across all replicates. Seed 8–12 plates per strain for lifespan experiments, and 2–4 plates per strain to grow animals to adulthood. Allow plates to dry overnight.
Add 100 μL of 10 mg/mL FUDR onto middle of bacterial lawn for the 8–12 plates that will be used for lifespan assay. Remember to leave 2–4 plates without FUDR as starter plates to allow animals to grow to adulthood. Let plates dry overnight. Safety: FUDR blocks DNA synthesis and thus it is recommended to wear gloves when handling.
Collect a synchronized population of C. elegans using a standard bleaching assay as described in section 1.3–1.4. Note: These animals need to be grown on plates without FUDR, as FUDR will cause animals to arrest/die.
Move 10–15 day 1 adult animals onto each of the 8–12 plates containing FUDR. For a standard lifespan, it is recommended to start with ~120 animals to ensure that sample size does not drop too far below 100 after censorship events (e.g., 8 plates of 15 animals = 120 animals; 12 plates of 10 animals = 120 animals). Note: Animals can also be moved onto FUDR at the L4 stage if it is imperative that progeny formation be completely avoided but animals must not be moved too early as this will cause animals to be at higher risk for vulval/intestinal protrusions and will increase censorship.
Lifespans are scored every other day until all animals have been scored as dead or censored. All dead or censored animals should be removed from the plate to avoid confusion and recounting the same animal. Note: for FUDR lifespans, any progeny can be ignored as they will arrest at the L1 stage and eventually die.
2.3. Lifespans using temperature sensitive sterile mutants.
Prepare NGM-agar plates by seeding plates with 100 μL bacteria of choice. For consistency, ensure that the same bacteria is used across all replicates. Seed 8–12 plates per strain for lifespan experiments, and 2–4 plates per strain to grow animals to adulthood. Let plates dry overnight.
Collect a synchronized population of C. elegans using a standard bleaching assay as described in section 1.3–1.4. Remember to grow animals at the restrictive temperature of 25 °C to ensure that animals are sterile.
Move 10–15 day 1 adult animals onto each of 8–12 plates. For a standard lifespan, it is recommended to start with ~120 animals to ensure that sample size does not drop too far below 100 after censorship events (e.g., 8 plates of 15 animals = 120 animals; 12 plates of 10 animals = 120 animals).
Lifespans are scored every other day until all animals have been scored as dead or censored. All dead or censored animals should be removed from the plate to avoid confusion and recounting the same animal. Note: when dealing with short-lived strains, it is recommended to score lifespans every day as lifespans at 25 °C are much shorter and thus the dynamic range is limited. In our experience, animals can be shifted back to 20 °C after day 2 and animals will remain sterile if it is preferable to score lifespans at 20 °C.
3. Measuring healthspan in C. elegans.
3.1. Measurements of locomotory behavior via thrashing.
Collect a synchronized population of C. elegans using a standard bleaching assay as described in section 1.3–1.4.
Move a small colony of day 1 adult worms onto an NGM-agar plate under a dissecting scope onto 10–20 μL of M9 solution. We recommend 10–15 animals as a manageable number of animals to count.
Focusing on one worm at a time, count the number of times the specimen switches from a concave to convex formation in 15 ss. It is recommended to use a hand counter and a timer so focus can be placed on the worm for the duration of the assay. Note: a video of the plate may be recorded for more thorough/easier analysis. For example, standard microscope eyepiece attachments are available for most smartphones and digital cameras (~$15–30), and these are a great option to video thrashing at a low cost.
Repeat step 3 for the other worms in the liquid, averaging out a total motility rate for 10–15 worms. For higher sample size, steps 2–4 can be repeated.
Age out worms to desired age. Similar methods for lifespan assays described in sections 2.1–2.3 can be used for aging out worms. Repeat steps 2–4 to assay thrashing at desired ages.
An alternative method for step 2 is to add ~30 μL or more of M9 solution onto a group of worms on a plate. This will save time from having to manually transfer worms, although due to random chance of where the worms are on a single plate, there is no guarantee that a group of worms will remain at a single point on the plate.
3.2. Measurements of fecundity (egg count) in C. elegans.
Collect a synchronized population of C. elegans using a standard bleaching assay as described in section 1.3–1.4. Assays for egg count start at the L4 stage, which is ~1 day prior to day 1 adulthood (~3 days at 15 °C or ~2 days at 20 °C after L1 arresting).
Single out L4 worms onto separate NGM-agar plates seeded with bacteria of choice. It is recommended that ~10–15 animals be used for a fecundity assay. Note: we recommend diluting the bacteria of choice by 50% (i.e., not a saturated culture) to improve the egg visibility in the bacterial lawn.
Allow animals to grow overnight at 20 °C. Ensure that a newly seeded batch of plates is ready for the next day.
On day 1 of adulthood, transfer adult worms onto fresh NGM-agar plates seeded with the diluted bacteria of choice. Note: it is recommended to use freshly seeded plates, or to store plates at 4 °C until use to prevent thick bacterial lawns.
Count the total number of eggs laid on each NGM-agar plate. Note: to aid in scanning the plate, a grid can be drawn on the lid of a plate and placed under the plate being scored for eggs. The plate can then be scanned along the grid lines to maintain orientation as the plate is moved and prevent recounting counting of any eggs.
Repeat steps 4–6 for 7–8 days or until eggs are no longer visible on the plate. Note: for days 1–3 when egg-laying rates are high, it is recommended to move animals at least every 12 hrs and assay egg counts twice a day. However, this increases the amount of work and costs of consumables, and thus moving animals and measurements can be limited to once per day, but care must be taken to ensure all eggs and hatched animals are counted properly. Note: Any hatched animals are counted as eggs for the purpose of this assay.
3.3. Measurement of brood size (development) of C. elegans progeny.
Collect a synchronized population of C. elegans using a standard bleaching assay as described in section 1.3–1.4. Assays for brood size start at the L4 stage, which is ~1 day prior to day 1 adulthood (~3 days at 15 °C or ~2 days at 20 °C after L1 arresting).
Single out L4 worms onto separate NGM-agar plates seeded with bacteria of choice. It is recommended that ~10–15 animals be used for a fecundity assay.
Allow animals to grow overnight at 20 °C. Ensure that a newly seeded batch of plates is ready for the next day.
On day 1 of adulthood, transfer adult worms onto fresh NGM-agar plates seeded with bacteria of choice.
Every 12–24 hrs (2x a day or 1x a day), transfer adult worms onto fresh NGM-agar plates seeded with bacteria of choice for 7–8 days or until progeny are no longer visible. Keep all the plates containing eggs at 20 °C.
Repeat step 5 for 7–8 days or until progeny are no longer visible. Keep all the plates containing eggs at 20 °C. Note: Progeny plates may also be stored at 15 °C to extend the time before they need to be scored.
Two days after transferring worms, count the developed progeny on the plates. It is recommended to count developing worms at the L4 stage (i.e., 2 days after hatch at 20 °C) or earlier to ensure that the F2 generation (i.e., progeny of progeny) does not confound results. Count all worms that are alive. Remove all worms from the plate as they are counted. Maintain the plates for an additional 1–2 days before scoring them again to ensure that any animals with delayed hatching/development are not missed.
Repeat step 6 for every egg-lay plate collected. Note: The brood size assays can be conducted in conjunction with egg count assay (section 3.2) to minimize labor and costs of consumables by collecting two sets of data from one experiment. This will also allow for direct comparison of brood size and egg count within the same animals.
4. Measuring stress resilience in C. elegans.
4.1. Measurements of ER stress sensitivity using tunicamycin.
Prepare NGM-agar plates by seeding plates containing tunicamycin (see section 1.1 step 7) with 100 μL bacteria of choice. Safety: gloves should be worn when handling tunicamycin.
For consistency, ensure that the same bacteria is used across all replicates. Seed 8–12 tunicamycin plates per strain for survival assays, and 2–4 plates without tunicamycin per strain to grow animals to adulthood. Let plates dry overnight.
Collect a synchronized population of C. elegans using a standard bleaching assay as described in section 1.3–1.4. Note: Animals must be grown on plates without tunicamycin until day 1 of adulthood, as animals will arrest/die on tunicamycin.
Move 10–15 day 1 adult animals onto each of 8–12 plates. For a standard survival assays, it is recommended to start with ~120 animals to ensure that sample size does not drop too far below 100 after censorship events (e.g., 8 plates of 15 animals = 120 animals; 12 plates of 10 animals = 120 animals). Note: similar to FUDR assays, tunicamycin survival assays can be performed without moving animals, as tunicamycin causes death/arrest of L1 animals. However, when performing a DMSO control, progeny will develop on DMSO plates, so animals need to be moved daily or a sterilization technique will be required (identical methods used in section 2 for lifespans can be used for survival assays).
Survival assays are scored similar to lifespans. All dead or censored animals should be removed from the plate to avoid confusion and recounting the same animal. Note: Although it is possible to score animals every other day, since death occurs rapidly on tunicamycin, we recommend scoring survival assays daily.
4.2. Measurements of mitochondrial/oxidative stress sensitivity using paraquat.
Prepare NGM-agar plates by seeding plates containing paraquat (see section 1.1 step 7) with 100 μL bacteria of choice. Safety: gloves should be worn when handling paraquat as it is an environmental hazard. Check with institution’s environmental health and safety for requirements of discarding as many research institutions will require specific discarding instructions for environmental hazards.
For consistency, ensure that the same bacteria is used across all replicates. Seed 8–12 plates per strain for survival assays, and 2–4 plates without paraquat per strain to grow animals to adulthood. Allow plates to dry overnight.
Collect a synchronized population of C. elegans using a standard bleaching assay as described in section 1.3–1.4. Note: Remember to grow animals on plates without paraquat until day 1 of adulthood; however, it is necessary to perform a sterilization technique or move adults away from progeny, as some animals can develop to adulthood on paraquat plates (see section 2.2–2.3,).
Move 10–15 day 1 adult animals onto each of 8–12 plates. For a standard survival assay, it is recommended to start with ~120 animals to ensure that sample size does not drop too far below 100 after censorship events (e.g., 8 plates of 15 animals = 120 animals; 12 plates of 10 animals = 120 animals).
Survival assays are scored similar to lifespans. All dead or censored animals should be removed from the plate to avoid confusion and recounting the same animal. Note: Although it is possible to score animals every other day, since death occurs rapidly on paraquat, we recommend scoring survival assays daily. This is especially true when using glp-4(bn2) animals grown at 25 °C as death will occur very rapidly.
4.3. Measurements of heat stress sensitivity (thermotolerance) using elevated temperatures.
Collect a synchronized population of C. elegans using a standard bleaching assay as described in section 1.3–1.4.
Pre-warm NGM agar plates to 37 °C prior to moving animals onto plate by placing plates into a 37 °C for at least one hour.
Move 10–15 day 1 adult animals onto each of 4–6 pre-warmed plates. For a standard thermotolerance, it is recommended to start with ~60 animals. For example, 4 plates of 15 animals = 60 animals; 6 plates of 10 animals = 60 animals.
Place animals into a 37 °C incubator and score for death every 2 hrs. Death is defined as animals that exhibit no movement when gently touched with a pick. All dead or censored animals should be removed from the plate to avoid confusion and recounting the same animal.
Ensure that plates are removed from the 37 °C for the minimal amount of time possible, as plates that are left in ambient temperature for long durations while scoring will alter thermotolerance results. Note: we recommend pulling out only 1 strain at a time to score, as the temperature of the agar should not change dramatically in the time it takes to score 1 strain.
Median thermotolerance is generally accomplished between 7–9 hrs, so ensure that hrs 7, 9, and 11 assayed properly. Note: while hrs 1–5 can be skipped, due to variability of incubators, thickness of plates, and other confounding factors in each lab, it is important that timing is titrated carefully in each lab if timepoints are planned to be skipped. See44 for a full guide on thermotolerance.
Alternatively, thermotolerance can be performed at 34 °C instead of 37 °C. Median thermotolerance at 34 °C occurs much later (~10–14 hrs in our hands), which allows for thermotolerance assays to be prepared late at night, placed into 34 °C, and scoring to begin early the next day. This allows for ~8 hrs of continued scoring rather than the typical 12-hrs required for a 37 °C thermotolerance assay.
REPRESENTATIVE RESULTS:
C. elegans are an excellent model organism for aging research due to a large majority of aging mechanisms being conserved with humans. Importantly, they have a very low cost in maintenance and experimentation with minimal requirements for equipment and consumables, making them a coveted model system for institutions with limited funds. Moreover, a plethora of simple assays with shallow learning curves make them an excellent system for even the youngest investigator with little to no experience. All these factors combined with the powerful genetics of C. elegans including the ease of genome editing, thousands of available mutants and transgenic animals at nominal costs, and available RNAi libraries for genetic knockdown of virtually every gene make them an ideal system for undergraduate institutions. Here, some of the lowest cost methods to study aging in C. elegans are surveyed, focusing primarily on assays with minimal equipment and consumable cost, as well as shallow learning curves. In fact, the entirety of the protocols and data collection were written/performed by junior investigators with <5 months of research experience, mostly undergraduate students.
Longevity studies in C. elegans are very simple due to the short lifespan of animals, ranging from 14–20 days. Importantly, lifespan assays are highly standardized and only require an incubator, a standard dissection microscope, a standard worm pick, and consumables for preparing NGM-agar plates. Perhaps the most cost-prohibitive aspect of lifespan measurements in C. elegans are consumables required. This is due to the fact that C. elegans are hermaphrodites that self-fertilize, therefore adults being tracked for longevity assays need to be moved away from progeny on a daily basis. However, animals can be sterilized by exposing them to FUDR or using the mutants, such as the temperature-sensitive germlineless glp-4(bn2) mutant grown at the prohibitive 25 °C to reduce the amount of consumables required30–32. Here, lifespan assays were performed with FUDR or with the glp-4(bn2) germlineless mutants, which show similar results to standard lifespans performed on non-sterile animals. While the wild-type lifespans are not identical due to the effects of FUDR45 or growth at 25 °C on lifespan2, the short-lived hsf-1 knockdown animal reliably shows a significant decrease in lifespan for all conditions (Fig. 1). hsf-1 encodes the heat-shock factor-1 transcription factor, which is involved in regulation of the thermal stress response, and its knockdown results in a significant decrease in lifespan38, 46.
Figure 1. Comparison of lifespan measurements with and without sterilization.
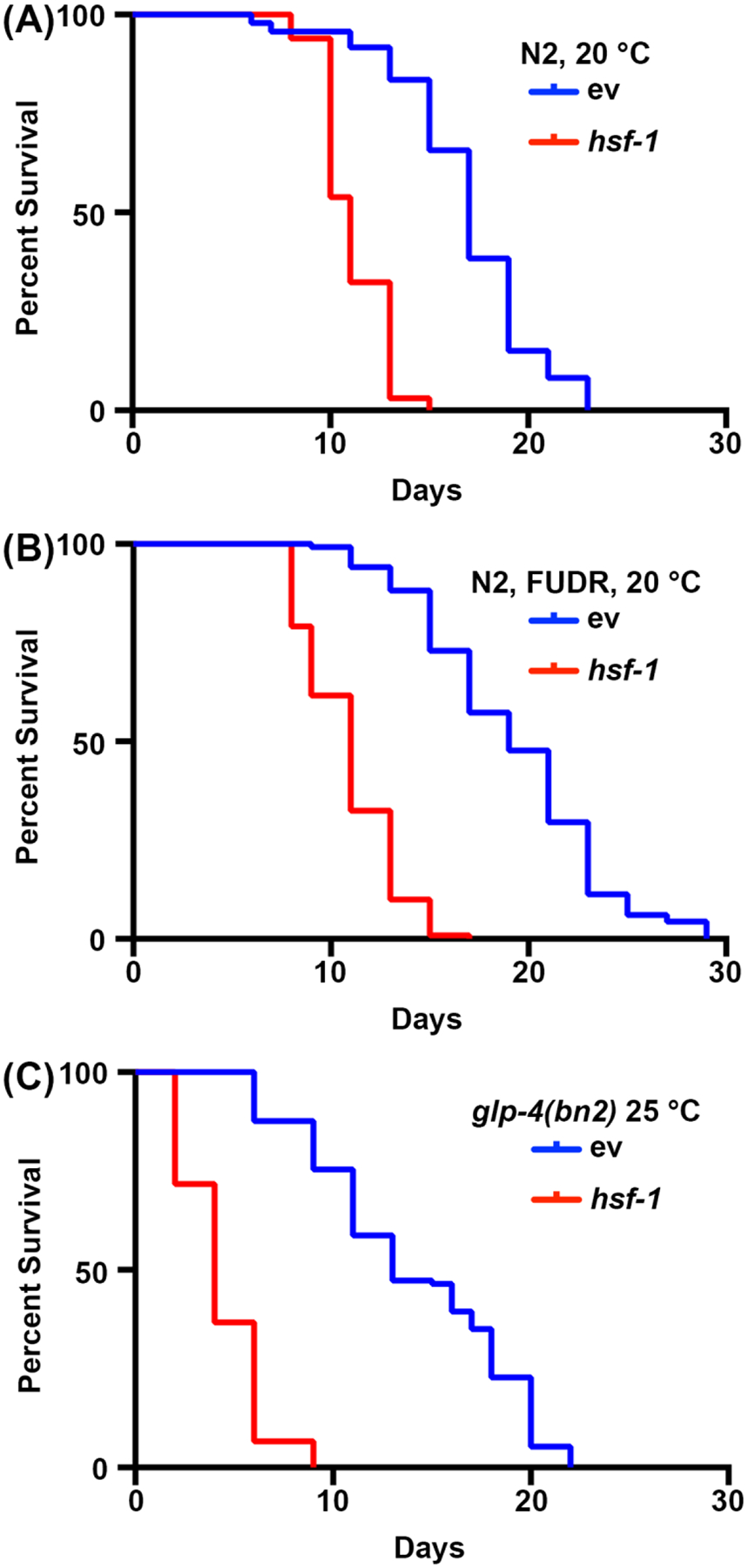
(A) Lifespans of wildtype N2 nematodes grown on NGM-agar plates seeded with empty vector (ev) or hsf-1 RNAi bacteria at 20 °C. Animals were moved away from progeny on day 1, 3, 5, and 7 of adulthood. (B) Lifespans of wildtype N2 nematodes grown on NGM-agar-FUDR plates seeded with empty vector (ev) or hsf-1 RNAi bacteria at 20 °C. Animals were grown to adulthood on standard ev or hsf-1 RNAi plates, then moved to FUDR plates at day 1 adulthood. (C) Lifespans of glp-4(bn2) mutant animals grown on NGM-agar plates seeded with empty vector (ev) or hsf-1 RNAi at 25 °C. For all conditions, animals were scored for death every 2 days until all animals were recorded as dead or censored. Animals with bagging, protrusion or explosion of vulva, or those that crawled up the sides of the plates and desiccated were censored. All statistics were performed using Log-Rank Mantel Cox testing and can be found in Table 2.
While longevity is an important factor to consider for aging biology, oftentimes longevity does not correlate with increased health, even in C. elegans47. Thus, as a complementary approach, we offer several methods of measuring organism health, including reproductive health, locomotory behavior, and stress resilience. Reproductive health can be measured in one of two ways. First, measurements of egg count will give a direct measurement of how many eggs are laid by a single self-fertilizing hermaphrodite. However, since animals produce more oocytes than sperm, some unfertilized eggs that would never produce viable progeny are also laid48. Therefore, to get a better understanding of the true reproductive capacity of an animal, measurements of the brood size provides a measure of how many viable offspring are produced. Often times, increased stress resilience can actually decrease reproductive capacity, potentially due to the inherent effect of perceived stress on reproduction49. Similarly, a significant decrease in both number of eggs laid and brood size is found in hsf-1 overexpression animals compared to wild-type controls (Fig. 2A–B). In fact, some hsf-1 overexpression animals exhibit full sterility, providing evidence that reproductive health can be inversely correlated with longevity.
Fig. 2. Egg count, brood size, and thrashing as measurements of healthspan.
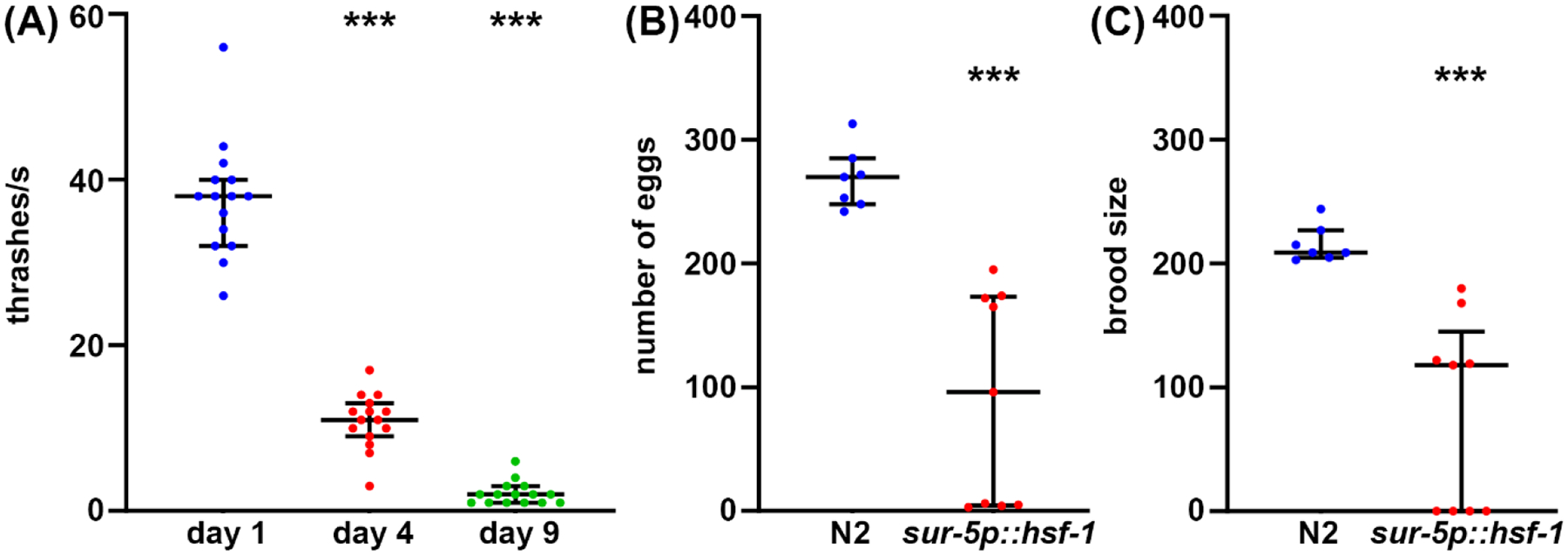
(A) Thrashing assays were performed on glp-4(bn2) mutant animals grown on NGM-agar plates seeded with empty vector at 25 °C on day 1 (blue), day 4 (red), and day 9 (green) animals. Thrashing was scored in animals placed into M9 solution on an NGM-agar plate, video recorded using a standard smartphone camera mounted onto an eyepiece of a standard dissecting scope, and thrashing scored in slow motion for accuracy. n = 15 animals per condition. (B) Egg counts were measured in wildtype N2 (blue) and sur-5p::hsf-1 (red) animals. Animals were grown at 20 °C and moved onto fresh plates and eggs counted every 12 hrs. Total number of eggs laid were summed. n = 7 animals for wildtype and 9 animals for sur-5p::hsf-1. (C) Brood assays were measured on the same animals as (B) where eggs were grown at 20 °C for two days to allow hatching, and all hatched eggs were counted. *** = p < 0.001 calculated using non-parametric Mann-Whitney testing. Each dot represents a single animal and lines represent median and interquartile range.
While reproductive health is important to understanding germline health, functional meiosis, and reproductive capacity, in general, there is no direct correlation between longevity and brood size50. Thus as a complementary approach, locomotory behavior is offered as a gold-standard method to assaying C. elegans healthspan during aging51. There are many methods to measure locomotory behavior, but most methods require sophisticated cameras, tracking software, or expensive chemicals. In contrast, thrashing assays require virtually no equipment beyond what a standard C. elegans lab is equipped with: dissecting microscope, worm pick, pipette, and consumables for making NGM-agar plates. Thrashing rates provide a reliable method for measuring healthspan during aging, as measured by a significant decrease in thrashing in old animals compared to young animals (Fig. 2C).
Finally, survival to stress assays are an additional physiological measurement of resilience. The capacity to activate stress responses generally declines during the aging process, making animals less resilient and more sensitive to stress. Thus, stress resilience can be used as a reliable proxy for organismal health. Here methods are offered for surveying sensitivity to 1) ER stress in response to tunicamycin exposure, a chemical agent that blocks N-linked glycosylation and results in accumulation of misfolded proteins in the ER; 2) mitochondrial/oxidative stress through exposure to paraquat, a chemical agent that induces superoxide formation in mitochondria; and 3) thermal stress through exposure to elevated temperatures. For tunicamycin and paraquat assays, the drug is incorporated into the NGM-agar plate during plate production. For high concentrations of tunicamycin, progeny generally do not develop, and thus sterilization techniques do not need to be used. The protocol presented here recommends 25 ng/μL as a final concentration of tunicamycin, but for those with limited funds, 10 ng/μL also shows significant reduction in survival (Fig. 3A). Both concentrations limit progeny development, and thus no sterilization methods are needed, although the DMSO control will require a sterilization technique or movement of animals onto new plates. This is because tunicamycin toxicity prevents development of progeny, but DMSO is virtually nontoxic, which allows progeny to develop fully when grown on tunicamycin.
Fig. 3. Stress resilience as a proxy to organismal health.
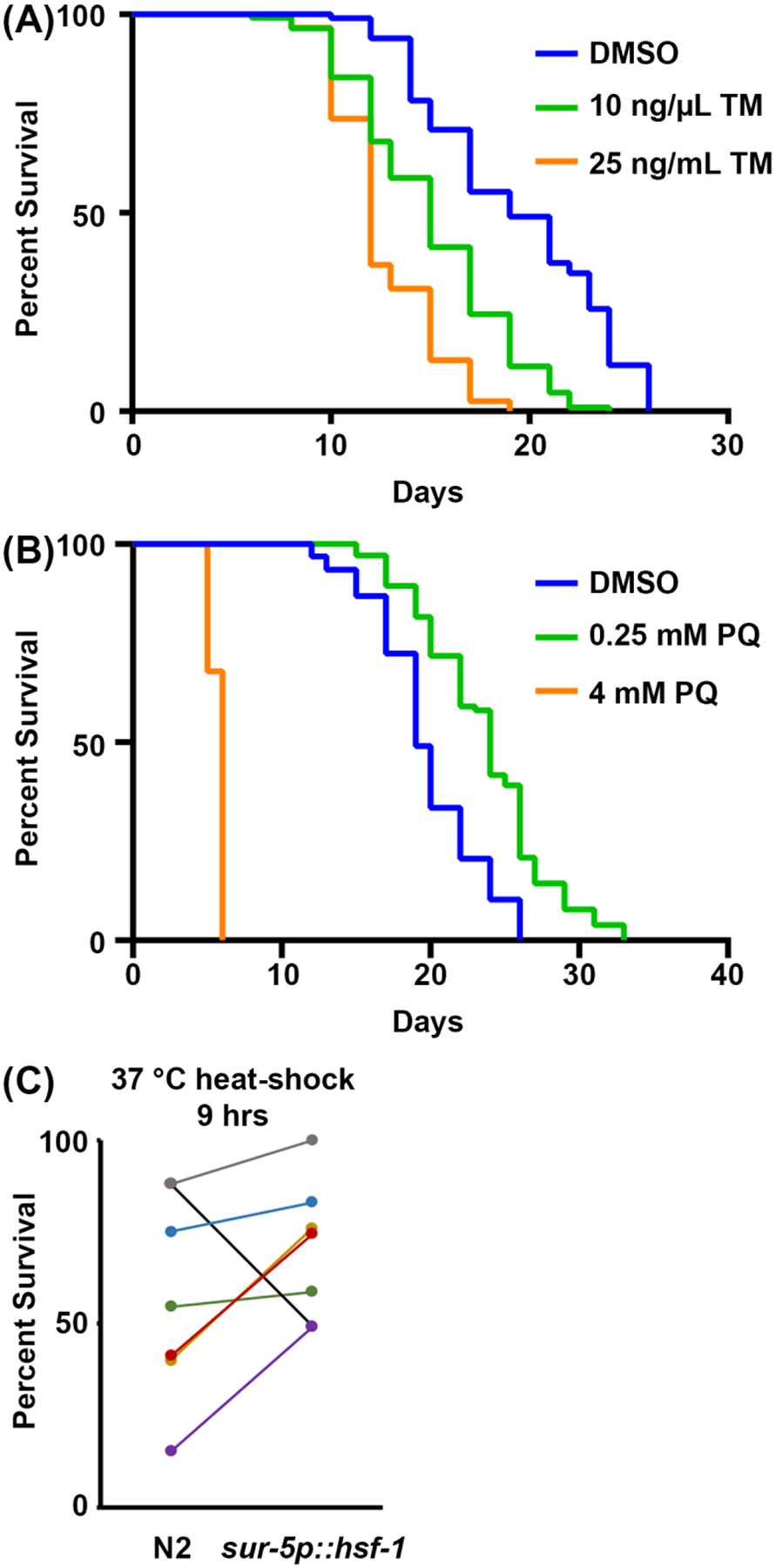
(A) Survival assay of N2 animals grown on empty vector (EV) RNAi bacteria at 20 °C. Animals were moved to either plates containing 1% DMSO, 10 ng/μL tunicamycin (TM), or 25 ng/μL TM on day 1 of adulthood. (B) Survival assay of N2 animals grown on empty vector (EV) RNAi bacteria at 20 °C. Animals were grown from hatch on plates containing either water, 0.25 mM paraquat (PQ), or 4 mM PQ. For A-B, animals were scored for death every 2 days until all animals were recorded as dead or censored. Animals with bagging, protrusion or explosion of vulva, or those that crawled up the sides of the plates and desiccated were censored. All statistics were performed using Log-Rank Mantel Cox testing and can be found in Table 2. (C) Pooled data of all thermotolerance assays performed at 37 °C for wild-type N2 animals versus overexpression of hsf-1 (sur-5p::hsf-1). Data are represented as percent alive at hr 9 of a thermotolerance assay with each line representing a matched experiment performed on the same day. Animals were grown on empty vector (ev) RNAi bacteria at 20 °C and moved to 37 °C at day 1 of adulthood for assay. n = 60 animals per strain per replicate.
For paraquat assays, either a sterilization technique or movement of animals is required as paraquat treatment does not prevent progeny from developing to adulthood. High levels of paraquat (4mM) significantly shortens lifespan, while low levels of paraquat (0.25 mM) actually increases lifespan due to a hormetic effect (Fig. 3B), consistent with previously published results52. Finally, thermotolerance assays only requires an incubator that can reach 30–37 °C and no additional reagents are required. Overexpression of hsf-1 increases thermotolerance at 37 °C (Fig. 3C) as previously published53. However, as the data presented here and others have shown previously, the major problem with thermotolerance assays is in its variability. There are many factors that can contribute to variability within thermotolerance assays, including differences between incubators and amount of time animals spend outside of the incubator while scoring thermotolerance each hour. For a thorough guideline of thermotolerance, refer to41.
Discussion:
Lifespan, most simply defined as the duration of life, is a clear binary phenomenon in most organisms – either an organism is living or is not. However, longevity does not always correlate with an organism’s health. For example, mitochondrial hormesis models where exposure to mitochondrial stress dramatically increases lifespan and are generally some of the longest-lived animals, yet exhibit stunted growth and decreased metabolic function37, 54. Similarly, animals with hyperactive endoplasmic reticulum stress responses also exhibit certain behaviors and phenotypes that can be correlated with decreased health, despite having dramatically improved protein homeostasis and lifespan36, 49. Finally, many longevity paradigms in model organisms including increased HSF-1 function55, increased XBP-1 function56, and altered FoxO signaling57 are all correlated with increased cancer risk, and it is inarguable that extended lifespan is not beneficial if an organism is in a constant struggle with cancer and other health maladies. Therefore, longevity cannot be a standalone measurement in aging biology.
Thus, the concept of healthspan has been a growing field in aging biology. Healthspan, loosely defined as the period of life that one is healthy, is more difficult to ascertain than longevity. However, unlike longevity, the concept of “health” is complicated, as there are many different readouts to organismal health: on the organismal level there are muscle function/strength, neuronal/cognitive function, reproductive health, etc.; on the cellular level there are protein homeostasis, lipid homeostasis, glucose homeostasis, metabolism, etc. In 2014, aging biologists have definitively characterized biological hallmarks of aging with the structured definition that it must be something that naturally breaks down during aging and can experimentally be altered such that experimental exacerbation should accelerate aging and experimental intervention should slow down aging. These nine hallmarks of aging include genomic instability, telomere attrition, epigenetic alterations, loss of protein homeostasis (proteostasis), stem cell exhaustion, altered intercellular signaling, mitochondrial dysfunction, deregulated nutrient sensing, and cellular senescence58. Since then, there are numerous studies that argue other factors should be included, including extracellular proteins and systemic physiology such as immunity and inflammation59. Ultimately, the complex definition of healthspan mandates that organismal health be measured using multiple different methods.
Therefore, in this manuscript, multiple methods are presented to measure various aspects of healthspan using the nematode model, C. elegans. We assay locomotory behavior using thrashing assays, reproductive health using egg count and brood size, and sensitivity to stress. Indeed, locomotory behavior is a gold-standard method for measuring healthspan, as organisms exhibit significant loss of motility and movement during aging51. Loss of locomotory behavior can be ascribed to multiple hallmarks of aging as muscle function in C. elegans is dependent on proper proteostasis60, mitochondrial dysfunction61, and neuron-muscle signaling62. While this manuscript focuses on one measurement of locomotory behavior, it is important to note that many other methods exist, including motility of animals on a solid agar plate, response to touch51, or chemotaxis assays63. However, these methods generally require more sophisticated recording devices, usage of worm-tracking software, or usage of expensive, dangerous, or volatile chemicals, all of which may be prohibitive in some research settings.
In addition, assays for egg count and brood size is presented as a method of reproductive health and as the simplest method to measure cell division in adult worms, since adult worms are post-mitotic and only germ cells and embryos undergo cell division within an adult worm64. As a measure of cell division, reproductive health can be relevant for the aging hallmarks of cellular senescence and stem cell exhaustion. Reproductive health can be affected by many factors, including pathogenic infection65 or exposure to stress49, though there is no direct correlation between reproductive health and longevity. In fact, some long-lived animals exhibit a significant decrease in brood size49, and it is even possible that there exist an inverse correlation between longevity and brood size50. This is not a phenomenon specific to C. elegans as detrimental effects of reproduction on longevity has long been observed in humans66, companion dogs67, and mice68. Still, we provide egg count and brood size as a reliable and low-cost method for measuring reproductive health with the caveat that reproductive health may not correlate with longevity or healthspan.
Finally, survival assays are offered as an indirect measure of organismal health. Importantly, cellular stress responses, including response to thermal stress69 and ER stress35 rapidly during the aging process and have direct relevance to the aging hallmark of proteostasis70, 71. In contrast, hyperactivating stress responses can significantly increase lifespan by promoting resilience to stress35, 37, 38. While this study focuses on the simplest and lowest cost methods, a large number of alternative methods for stress resilience assays exist for thermotolerance41 and oxidative stress66, each requiring a different set of equipment and consumables. Beyond simple exposure studies to stressors, other physiological methods can be performed depending on access to equipment. For example, those with a Seahorse XFp Analyzer can monitor mitochondrial function and cellular respiration73; fluorescent dissection microscopes will allow measurements of transcriptional reporters for stress response activation20; and high resolution compound or confocal microscopes can be used to measure organelle morphology with fluorescent probes for mitochondria74, the endoplasmic reticulum75,76, and actin cytoskeleton77.
As a final cautionary tale for measurements of longevity, while chemical and genetic methods for sterilizing worms are offered to significantly decrease cost, it is important to note that both can directly impact lifespan. For example, exposure to FUDR has been previously reported to impact both lifespan and thermotolerance45. And while the glp-4(bn2) mutant itself does not have any direct effects on lifespan, growth at 25 °C is a mild heat-stress33, 34 and thus can impact lifespan2. There exist other methods for sterilizing C. elegans including auxin-mediated sterility78 or alternative temperature sensitive sperm-deficient mutants79. However, all methods have some caveats, and care should be taken to utilize the least detrimental assay for each laboratory’s scientific needs. One final limitation of longevity studies is potential variability that can occur due to low sample sizes or simply by objective error by the investigator. This can be circumvented as new technologies are born in automated lifespan technologies80, but again these systems are costly and require some engineering and computational equipment and skills. Ultimately, the collection of methods provided here are a reliable set of tools that can be quickly adapted and learned in almost any institution and provide a solid foundation for aging biology.
Table 2.
Statistics for lifespan and stress resilience.
| Corresponding Figure | Strain, Treatment | Median Lifespan | # Deaths/# Total | p-value (Log-Rank) |
|---|---|---|---|---|
| 1A | N2, vector RNAi, 20 °C | 17 | 74/120 | -- |
| N2, hsf-1 RNAi, 20 °C | 11 | 65/120 | <0.001 | |
| 1B | N2, vector RNAi, FUDR, 20 °C | 19 | 120/120 | -- |
| N2, hsf-1 RNAi, FUDR, 20 °C | 11 | 116/120 | <0.001 | |
| 1C | N2, glp-4(bn2), vector RNAi, 25 °C | 13 | 115/121 | -- |
| N2, glp-4(bn2), hsf-1 RNAi, 25 °C | 4 | 120/120 | < 0.001 | |
| 2A | N2, vector RNAi, 20 °C, 1% DMSO | 19 | 85/120 | -- |
| N2, vector RNAi, 20 °C, 10 ng/μL tunicamycin | 15 | 109/120 | <0.001 | |
| N2, vector RNAi, 20 °C, 25 ng/μL tunicamycin | 12 | 117/120 | <0.001 | |
| 2B | N2, vector RNAi, 20 °C | 19 | 84/120 | -- |
| N2, vector RNAi, 20 °C, 0.25 mM paraquat | 24 | 91/120 | <0.001 | |
| N2, vector RNAi, 20 °C, 4 mM paraquat | 6 | 50/120 | <0.001 |
ACKNOWLEDGEMENTS:
G.G. is supported by T32AG052374 and R.H.S. is supported by R00AG065200 from the National Institute on Aging. We thank the CGC (funded by NIH Office of Research Infrastructure Program P40 OD010440) for strains.
Footnotes
A complete version of this article that includes the video component is available at http://dx.doi.org/10.3791/64091.
DISCLOSURES:
The authors have nothing to disclose.
REFERENCES:
- 1.Brenner S The genetics of Caenorhabditis elegans. Genetics. 77 (1), 71–94, doi: 10.1093/genetics/77.1.71 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klass MR Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 6 (6), 413–29, doi: 10.1016/0047-6374(77)90043-4 (1977). [DOI] [PubMed] [Google Scholar]
- 3.Friedman DB, Johnson TE A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics. 118 (1), 75–86, doi: 10.1093/genetics/118.1.75 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R A C. elegans mutant that lives twice as long as wild type. Nature. 366 (6454), 461–4, doi: 10.1038/366461a0 (1993). [DOI] [PubMed] [Google Scholar]
- 5.Lithgow GJ, White TM, Melov S, Johnson TE Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proceedings of the National Academy of Sciences of the United States of America. 92 (16), 7540–7544, doi: 10.1073/pnas.92.16.7540 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epel ES, Lithgow GJ Stress biology and aging mechanisms: toward understanding the deep connection between adaptation to stress and longevity. J Gerontol A Biol Sci Med Sci. 69 Suppl 1 (Suppl 1), S10–6, doi: 10.1093/gerona/glu055 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luo Y Long-lived worms and aging. Redox Rep. 9 (2), 65–9, doi: 10.1179/135100004225004733 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Tissenbaum HA Using C. elegans for aging research. Invertebrate reproduction & development. 59 (sup1), 59–63, doi: 10.1080/07924259.2014.940470 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang S, Li F, Zhou T, Wang G, Li Z Caenorhabditis elegans as a Useful Model for Studying Aging Mutations. 11 (731), doi: 10.3389/fendo.2020.554994 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner S The genetics of Caenorhabditis elegans. Genetics. 77 (1), 71–94 (1974). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rual J-F et al. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Research. 14 (10B), 2162–2168, doi: 10.1101/gr.2505604 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Timmons L, Court DL, Fire A Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene. 263 (1–2), 103–112, doi: 10.1016/s0378-1119(00)00579-5 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Reboul J et al. Open-reading-frame sequence tags (OSTs) support the existence of at least 17,300 genes in C. elegans. Nature Genetics. 27 (3), 332–336, doi: 10.1038/85913 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Lee SS, Lee RYN, Fraser AG, Kamath RS, Ahringer J, Ruvkun G A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nature Genetics. 33 (1), 40–48, doi: 10.1038/ng1056 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Reinke SN, Hu X, Sykes BD, Lemire BD Caenorhabditis elegans diet significantly affects metabolic profile, mitochondrial DNA levels, lifespan and brood size. Molecular Genetics and Metabolism. 100 (3), 274–282, doi: 10.1016/j.ymgme.2010.03.013 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Revtovich AV, Lee R, Kirienko NV Interplay between mitochondria and diet mediates pathogen and stress resistance in Caenorhabditis elegans. PLOS Genetics. 15 (3), e1008011, doi: 10.1371/journal.pgen.1008011 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pang S, Curran SP Adaptive capacity to bacterial diet modulates aging in C. elegans. Cell Metabolism. 19 (2), 221–231, doi: 10.1016/j.cmet.2013.12.005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks KK, Liang B, Watts JL The Influence of Bacterial Diet on Fat Storage in C. elegans. PLOS ONE. 4 (10), e7545, doi: 10.1371/journal.pone.0007545 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soukas AA, Kane EA, Carr CE, Melo JA, Ruvkun G Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes & Development. 23 (4), 496–511, doi: 10.1101/gad.1775409 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bar-Ziv R et al. Measurements of Physiological Stress Responses in C. Elegans. JoVE (Journal of Visualized Experiments). (159), e61001, doi: 10.3791/61001 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xiao R, Chun L, Ronan EA, Friedman DI, Liu J, Xu XZS RNAi Interrogation of Dietary Modulation of Development, Metabolism, Behavior, and Aging in C. elegans. Cell Reports. 11 (7), 1123–1133, doi: 10.1016/j.celrep.2015.04.024 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calixto A, Chelur D, Topalidou I, Chen X, Chalfie M Enhanced neuronal RNAi in C. elegans using SID-1. Nature Methods. 7 (7), 554–559, doi: 10.1038/nmeth.1463 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dickinson DJ, Goldstein B CRISPR-Based Methods for Caenorhabditis elegans Genome Engineering. Genetics. 202 (3), 885–901, doi: 10.1534/genetics.115.182162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim H-M, Colaiácovo MP CRISPR-Cas9-Guided Genome Engineering in C. elegans. Current protocols in molecular biology. 129 (1), e106, doi: 10.1002/cpmb.106 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farboud B, Severson AF, Meyer BJ Strategies for Efficient Genome Editing Using CRISPR-Cas9. Genetics. 211 (2), 431–457, doi: 10.1534/genetics.118.301775 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Evans TC Transformation and microinjection. WormBook: The Online Review of C. elegans Biology [Internet]. at <https://www.ncbi.nlm.nih.gov/books/NBK19648/>. WormBook. (2006).
- 27.Frøkjaer-Jensen C et al. Single-copy insertion of transgenes in Caenorhabditis elegans. Nature Genetics. 40 (11), 1375–1383, doi: 10.1038/ng.248 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaymak E et al. Efficient generation of transgenic reporter strains and analysis of expression patterns in Caenorhabditis elegans using Library MosSCI. Developmental dynamics : an official publication of the American Association of Anatomists. 245 (9), 925–936, doi: 10.1002/dvdy.24426 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mariol M-C, Walter L, Bellemin S, Gieseler K A Rapid Protocol for Integrating Extrachromosomal Arrays With High Transmission Rate into the C. elegans Genome. Journal of Visualized Experiments : JoVE. (82), doi: 10.3791/50773 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rastogi S et al. Caenorhabditis elegans glp-4 Encodes a Valyl Aminoacyl tRNA Synthetase. G3: Genes|Genomes|Genetics. 5 (12), 2719–2728, doi: 10.1534/g3.115.021899 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beanan MJ, Strome S Characterization of a germ-line proliferation mutation in C. elegans. Development (Cambridge, England). 116 (3), 755–766, doi: 10.1242/dev.116.3.755 (1992). [DOI] [PubMed] [Google Scholar]
- 32.Santi DV, McHenry CS 5-Fluoro-2ʹ-Deoxyuridylate: Covalent Complex with Thymidylate Synthetase. Proceedings of the National Academy of Sciences. 69 (7), 1855–1857, doi: 10.1073/pnas.69.7.1855 (1972). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lithgow GJ, White TM, Hinerfeld DA, Johnson TE Thermotolerance of a long-lived mutant of Caenorhabditis elegans. Journal of Gerontology. 49 (6), B270–276, doi: 10.1093/geronj/49.6.b270 (1994). [DOI] [PubMed] [Google Scholar]
- 34.Labbadia J, Morimoto RI The Biology of Proteostasis in Aging and Disease. Annual Review of Biochemistry. 84 (1), 435–464, doi: 10.1146/annurev-biochem-060614-033955 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor RC, Dillin A XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell. 153 (7), 1435–1447, doi: 10.1016/j.cell.2013.05.042 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higuchi-Sanabria R et al. Divergent Nodes of Non-autonomous UPRER Signaling through Serotonergic and Dopaminergic Neurons. Cell Reports. 33 (10), 108489, doi: 10.1016/j.celrep.2020.108489 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durieux J, Wolff S, Dillin A The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 144 (1), 79–91, doi: 10.1016/j.cell.2010.12.016 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morley JF, Morimoto RI Regulation of longevity in Caenorhabditis elegans by heat shock factor and molecular chaperones. Molecular Biology of the Cell. 15 (2), 657–664, doi: 10.1091/mbc.E03-07-0532 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heifetz A, Keenan RW, Elbein AD Mechanism of action of tunicamycin on the UDP-GlcNAc:dolichyl-phosphate Glc-NAc-1-phosphate transferase. Biochemistry. 18 (11), 2186–2192, doi: 10.1021/bi00578a008 (1979). [DOI] [PubMed] [Google Scholar]
- 40.Castello PR, Drechsel DA, Patel M Mitochondria are a major source of paraquat-induced reactive oxygen species production in the brain. The Journal of Biological Chemistry. 282 (19), 14186–14193, doi: 10.1074/jbc.M700827200 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park H-EH, Jung Y, Lee S-JV Survival assays using Caenorhabditis elegans. Molecules and Cells. 40 (2), 90–99, doi: 10.14348/molcells.2017.0017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardner M, Rosell M, Myers EM Measuring the Effects of Bacteria on C. Elegans Behavior Using an Egg Retention Assay. Journal of Visualized Experiments : JoVE. (80), 51203, doi: 10.3791/51203 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waggoner LE, Hardaker LA, Golik S, Schafer WR Effect of a Neuropeptide Gene on Behavioral States in Caenorhabditis elegans Egg-Laying. Genetics. 154 (3), 1181–1192 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zevian SC, Yanowitz JL Methodological Considerations for Heat Shock of the Nematode Caenorhabditis elegans. Methods (San Diego, Calif.). 68 (3), 450–457, doi: 10.1016/j.ymeth.2014.04.015 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feldman N, Kosolapov L, Ben-Zvi A Fluorodeoxyuridine Improves Caenorhabditis elegans Proteostasis Independent of Reproduction Onset. PLOS ONE. 9 (1), e85964, doi: 10.1371/journal.pone.0085964 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hsu A-L, Murphy CT, Kenyon C Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science (New York, N.Y.). 300 (5622), 1142–1145, doi: 10.1126/science.1083701 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Bansal A, Zhu LJ, Yen K, Tissenbaum HA Uncoupling lifespan and healthspan in Caenorhabditis elegans longevity mutants. Proceedings of the National Academy of Sciences of the United States of America. 112 (3), E277–286, doi: 10.1073/pnas.1412192112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hodgkin J, Barnes TM More is not better: brood size and population growth in a self-fertilizing nematode. Proceedings. Biological Sciences 246 (1315), 19–24, doi: 10.1098/rspb.1991.0119 (1991). [DOI] [PubMed] [Google Scholar]
- 49.Özbey NP et al. Tyramine Acts Downstream of Neuronal XBP-1s to Coordinate Inter-tissue UPRER Activation and Behavior in C. elegans. Developmental Cell. 55 (6), 754–770.e6, doi: 10.1016/j.devcel.2020.10.024 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee Y et al. Inverse correlation between longevity and developmental rate among wild C. elegans strains. Aging (Albany NY). 8 (5), 986–994, doi: 10.18632/aging.100960 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swierczek NA, Giles AC, Rankin CH, Kerr RA High-throughput behavioral analysis in C. elegans. Nature Methods. 8 (7), 592–598, doi: 10.1038/nmeth.1625 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S-J, Hwang AB, Kenyon C Inhibition of respiration extends C. elegans life span via reactive oxygen species that increase HIF-1 activity. Current biology: CB. 20 (23), 2131–2136, doi: 10.1016/j.cub.2010.10.057 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baird NA et al. HSF-1-mediated cytoskeletal integrity determines thermotolerance and life span. Science (New York, N.Y.). 346 (6207), 360–363, doi: 10.1126/science.1253168 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Houtkooper RH et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 497 (7450), 451–457, doi: 10.1038/nature12188 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carpenter RL, Gökmen-Polar Y HSF1 as a Cancer Biomarker and Therapeutic Target. Current cancer drug targets. 19 (7), 515–524, doi: 10.2174/1568009618666181018162117 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen S et al. The emerging role of XBP1 in cancer. Biomedicine & Pharmacotherapy. 127, 110069, doi: 10.1016/j.biopha.2020.110069 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Yadav RK, Chauhan AS, Zhuang L, Gan B FoxO transcription factors in cancer metabolism. Seminars in cancer biology. 50, 65–76, doi: 10.1016/j.semcancer.2018.01.004 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G The hallmarks of aging. Cell. 153 (6), 1194–1217, doi: 10.1016/j.cell.2013.05.039 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennedy BK et al. Geroscience: linking aging to chronic disease. Cell. 159 (4), 709–713, doi: 10.1016/j.cell.2014.10.039 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ben-Zvi A, Miller EA, Morimoto RI Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proceedings of the National Academy of Sciences. 106 (35), 14914–14919, doi: 10.1073/pnas.0902882106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hewitt JE et al. Muscle strength deficiency and mitochondrial dysfunction in a muscular dystrophy model of Caenorhabditis elegans and its functional response to drugs. Disease Models & Mechanisms. 11 (12), dmm036137, doi: 10.1242/dmm.036137 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao S, Zhen M Action potentials drive body wall muscle contractions in Caenorhabditis elegans. Proceedings of the National Academy of Sciences of the United States of America. 108 (6), 2557–2562, doi: 10.1073/pnas.1012346108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Margie O, Palmer C, Chin-Sang IC elegans Chemotaxis Assay. Journal of Visualized Experiments : JoVE. (74), 50069, doi: 10.3791/50069 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flemming AJ, Shen ZZ, Cunha A, Emmons SW, Leroi AM Somatic polyploidization and cellular proliferation drive body size evolution in nematodes. Proceedings of the National Academy of Sciences of the United States of America. 97 (10), 5285–5290, doi: 10.1073/pnas.97.10.5285 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Madhu BJ, Salazar AE, Gumienny TL Caenorhabditis elegans egg-laying and brood-size changes upon exposure to Serratia marcescens and Staphylococcus epidermidis are independent of DBL-1 signaling. microPublication Biology. 2019, 10.17912/2r51-b476, doi: 10.17912/2r51-b476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Westendorp RG, Kirkwood TB Human longevity at the cost of reproductive success. Nature. 396 (6713), 743–746, doi: 10.1038/25519 (1998). [DOI] [PubMed] [Google Scholar]
- 67.Hoffman JM, Creevy KE, Promislow DEL Reproductive Capability Is Associated with Lifespan and Cause of Death in Companion Dogs. PLOS ONE. 8 (4), e61082, doi: 10.1371/journal.pone.0061082 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garratt M, Try H, Smiley KO, Grattan DR, Brooks RC Mating in the absence of fertilization promotes a growth-reproduction versus lifespan trade-off in female mice. Proceedings of the National Academy of Sciences of the United States of America. 117 (27), 15748–15754, doi: 10.1073/pnas.2003159117 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Labbadia J, Morimoto RI Repression of the Heat Shock Response Is a Programmed Event at the Onset of Reproduction. Mol Cell. 59 (4), 639–50, doi: 10.1016/j.molcel.2015.06.027 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Higuchi-Sanabria R, Frankino PA, Paul JW, Tronnes SU, Dillin A A Futile Battle? Protein Quality Control and the Stress of Aging. Developmental Cell. 44 (2), 139–163, doi: 10.1016/j.devcel.2017.12.020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dutta N, Garcia G, Higuchi-Sanabria R Hijacking Cellular Stress Responses to Promote Lifespan. Frontiers in Aging. 3, at <https://www.frontiersin.org/article/10.3389/fragi.2022.860404> (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Senchuk MM, Dues DJ, Van Raamsdonk JM Measuring Oxidative Stress in Caenorhabditis elegans: Paraquat and Juglone Sensitivity Assays. Bio-protocol. 7 (1), doi: 10.21769/BioProtoc.2086 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leung DTH, Chu S Measurement of Oxidative Stress: Mitochondrial Function Using the Seahorse System. Methods in molecular biology (Clifton, N.J.). 1710, 285–293, doi: 10.1007/978-1-4939-7498-6_22 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Daniele JR, Esping DJ, Garcia G, Parsons LS, Arriaga EA, Dillin A High-Throughput Characterization of Region-Specific Mitochondrial Function and Morphology. Scientific Reports. 7 (1), 6749, doi: 10.1038/s41598-017-05152-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu N et al. The FATP1–DGAT2 complex facilitates lipid droplet expansion at the ER–lipid droplet interface. The Journal of Cell Biology. 198 (5), 895–911, doi: 10.1083/jcb.201201139 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Daniele JR et al. UPRER promotes lipophagy independent of chaperones to extend life span. Science Advances. 6 (1), eaaz1441, doi: 10.1126/sciadv.aaz1441 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Higuchi-Sanabria R et al. Spatial regulation of the actin cytoskeleton by HSF-1 during aging. Molecular Biology of the Cell. 29 (21), 2522–2527, doi: 10.1091/mbc.E18-06-0362 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kasimatis KR, Moerdyk-Schauwecker MJ, Phillips PC Auxin-Mediated Sterility Induction System for Longevity and Mating Studies in Caenorhabditis elegans. G3: Genes|Genomes|Genetics. 8 (8), 2655–2662, doi: 10.1534/g3.118.200278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fabian TJ, Johnson TE Production of age-synchronous mass cultures of Caenorhabditis elegans. Journal of Gerontology. 49 (4), B145–156, doi: 10.1093/geronj/49.4.b145 (1994). [DOI] [PubMed] [Google Scholar]
- 80.Stroustrup N, Ulmschneider BE, Nash ZM, López-Moyado IF, Apfeld J, Fontana W The Caenorhabditis elegans Lifespan Machine. Nature Methods. 10 (7), 665–670, doi: 10.1038/nmeth.2475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]


