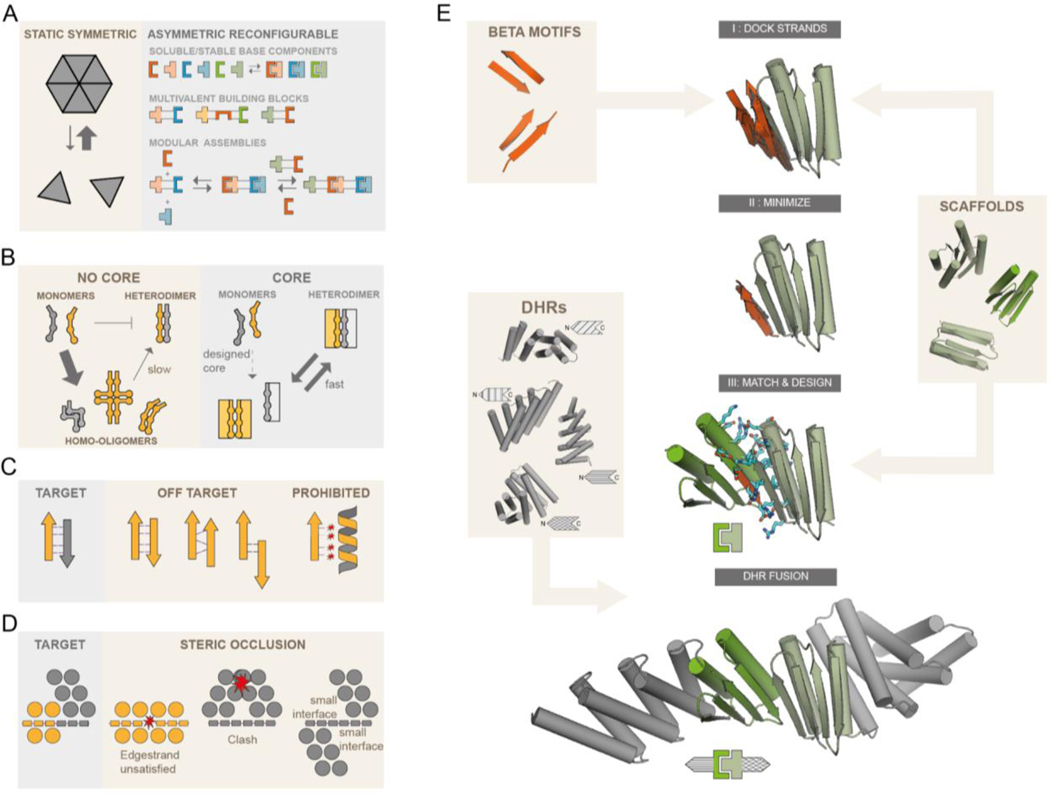
Fig. 1. Strategies for the design of asymmetric hetero-oligomeric complexes.
(A) Many design efforts have focused on cooperatively assembling symmetric complexes (left) with little subunit exchange. Here we sought to create asymmetric hetero-oligomers from stable heterodimeric building blocks, that can modularly exchange subunits (right). (B,C,D) Schematic illustration of properties that can contribute to preventing self-association. (B) Protomers that have a substantial hydrophobic core (right rectangles) are less likely to form stable homo-oligomers than protomers of previously designed heterodimers lacking hydrophobic monomer cores. (C) In beta-sheet extended interfaces, most homodimer states that bury non h-bonding polar edge strand atoms are energetically inaccessible. Potential homodimers are more likely to form via beta sheet extension. These are restricted to only 2 orientations (parallel and antiparallel) and a limited number of offset registers. Arrows and ribbons represent strands and helices, respectively; thin lines indicate hydrogen bonds, red stars indicate unsatisfied polar groups. (D) “Cross sectional” schematic view (helices as circles, beta strands as rectangles, star indicates steric clash) By modeling the limited number of beta sheet homodimers across the beta edge strand, structural elements may be designed that specifically block homodimer formation or make it unlikely due to small interfaces, but still allow heterodimer formation. (E) Design workflow: Beta sheet motifs are docked to the edge strands of a library of hydrophobic core containing (modified) fold-it scaffolds. Minimized docked strands are incorporated into the scaffolds by matching the strands to the scaffold library, yielding docked protein-protein complexes, followed by interface sequence design. Resulting docks are fused rigidly on their terminal helices to a library of DHRs.