Abstract
Repetitive negative thinking (RNT) represents a transdiagnostic risk factor for affective disorders, and stress is theorized to exacerbate this vulnerability. One mechanism by which stress may influence individual differences in psychiatric symptoms is through altered decision-making, and loss aversion in particular. The present study uses multiple methods to investigate the relationships between RNT, stress, and decision-making. We measured RNT in young adults (N = 90) recently exposed to a natural stressor, Hurricane Irma, and tested the influence of RNT on changes in affect, cortisol, and decision-making during a laboratory stress induction two months later. Post-hurricane RNT predicted greater increases in loss averse decision-making (β = 0.30 [0.14, 0.47], p < .001; rp2 = 0.079) and negative affect (β = 0.59 [0.37, 0.81], p < .001; rp2 = 0.319) during the early-phase response to the laboratory stressor, as well as poorer cortisol recovery (β = 0.32, [0.10, 0.54], p = .005; rp2 = 0.095) in the late-phase stress response. Results highlight the role of loss aversion and stress in understanding RNT as an affective vulnerability factor.
Keywords: Repetitive Negative thinking, Decision-making, Loss aversion, Stress, Cortisol
1. Introduction
Anxiety and depressive disorders impact approximately 2.5 billion individuals (World Health Organization, 2017). The global burden of these disorders necessitates research on vulnerability factors such as negative cognitive styles or processing biases (Nolen-Hoeksema, Wisco, & Lyubomirsky, 2008). While future-focused, negative cognitive styles tend to manifest as worry and predict anxiety; past-focused negative cognitive styles manifest as rumination and predict depression (Nolen-Hoeksema et al., 2008). Notably, rumination and worry contribute not only to depression and anxiety, but rather to myriad co-occurring psychiatric disorders (Hartley, Haddock, Vasconcelos E Sa, Emsley, & Barrowclough, 2014; McLaughlin & Nolen-Hoeksema, 2011). This has shaped the construct of repetitive negative thinking (RNT), a transdiagnostic process capturing overlap between worry and rumination (Ehring & Watkins, 2008). RNT has been prospectively linked with depression and anxiety disorders (Spinhoven, van Hemert, & Penninx, 2018) and cross-sectionally tied to obsessive-compulsive, body dysmorphic, and panic symptoms (Arditte, Shaw, & Timpano, 2016). While these findings support RNT as a transdiagnostic cognitive risk factor, high RNT does not singularly engender psychiatric symptom development (Calmes & Roberts, 2007). This suggests a need to identify behavioral, affective, and physiological correlates of RNT, particularly under conditions of stress, that may influence individual differences in symptom development.
Stressful life events exacerbate risk for psychiatric diagnoses, including post-traumatic stress disorder (Sutker, Corrigan, Sundgaard-Riise, Uddo, & Allain, 2002), mood and anxiety disorders (Robinson & Alloy, 2003), and schizophrenia (Howes et al., 2004). These findings underlie diathesis-stress models, which posit stress as an “activating” ingredient in psychopathology development that accounts for unexplained variance in cognitive models (Beck, Rush, Shaw, & Emery, 1979). Supporting the diathesis-stress framework, research indicates that individuals with cognitive vulnerabilities who experience stressful life events are more likely to develop psychopathology (Gibb & Coles, 2005). For RNT-related processes (e.g., worry and rumination), diathesis-stress effects are significant beyond the effects of cognitive vulnerabilities on psychopathology alone (Robinson & Alloy, 2003). Consequently, better understanding the relationship of transdiagnostic RNT to stress—through both naturalistic and laboratory paradigms—may bridge the aforementioned gaps in models of individual differences.
Modeling stress responses in a controlled laboratory setting allows researchers to test specific components of the cognitive diathesis-stress model. Acute laboratory stressors such as the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993) offer a controlled measure of individual variation in physiological stress responses. Though removed from a naturalistic setting, the TSST reliably elicits greater stress reactivity (e.g., cortisol and mood changes) in clinical samples compared to controls (Allen, Kennedy, Cryan, Dinan, & Clarke, 2014). Moreover, the TSST makes it possible to capture stress reactivity with high temporal resolution, permitting researchers to identify the time course of stress responses before, during, and after the onset of the stressor. This is critical for understanding the relationship of stress with transdiagnostic risk factors such as RNT, which could influence both the early-phase, or anticipation, and late-phase, or recovery, of responses to stress (e.g., in worry and rumination, respectively; Capobianco, Morris, & Wells, 2018).
Several experimental studies have assessed the effects of disorder-specific RNT measures (e.g., rumination, worry) on responses to stressors such as the TSST (e.g., Nolen-Hoeksema et al., 2008). However, only one has examined cognitive diathesis-stress relationships from a transdiagnostic perspective (Kertz, Stevens, & Klein, 2017), and none have linked RNT in the wake of a naturalistic stressor with later stress responding. The sole experimental study on RNT and stress found that high-RNT individuals displayed increased negative affect in response to stress (Kertz et al., 2017). Beyond examining self-reported affect as a measure of stress reactivity, laboratory measures of stress afford researchers the opportunity to assess stress-related changes in cognitive and physiological process. By linking these levels of analysis, researchers may pinpoint likely mechanisms by which cognitive vulnerabilities lead to psychiatric symptoms during both early- and late-phase responses to stressful life events. However, there is virtually no extant work relating RNT with stress responses in the context of both laboratory and real-world stress.
An additional cognitive process that may be central to transdiagnostic psychiatric risk, particularly under stressful conditions, is aberrant decision-making (Bishop & Gagne, 2018). Greater risk aversion, defined as the preference for certain over uncertain outcomes with comparable expected values (Charpentier, Aylward, Roiser, & Robinson, 2017), has been observed in certain anxiety disorders and under non-stressful neutral conditions (Giorgetta et al., 2012). Importantly, however, the majority of studies on decision-making and psychopathology have focused exclusively on risk aversion. Previous work has largely neglected loss aversion and choice consistency, two additional relevant facets of decision-making that may be differentially impaired in disorders where RNT is elevated (Charpentier et al., 2017; Charpentier, Martino, Sim, Sharot, & Roiser, 2015).
Loss aversion, or an unwillingness to entertain potential losses when choosing between options, may be especially related to individual differences in risk for internalizing symptoms, because it reflects heightened punishment sensitivity (Sokol-Hessner & Rutledge, 2011). Individuals with internalizing psychopathology, and depression in particular, display greater sensitivity to losses as well as higher learning rates after a loss compared with a win (Engelmann, Berns, & Dunlop, 2017). Further, neuroimaging evidence points to loss aversion as a potential neurobiological marker of depression, with greater activity in the midbrain region ventral tegmental area after losses in patients with depression compared to healthy controls (Chandrasekhar Pammi et al., 2015). Critically, no studies have assessed the relationship of loss aversion with a transdiagnostic risk factor for internalizing psychopathology (RNT) in non-clinical samples (Spinhoven et al., 2018), an important step in understanding whether loss aversion is a potential premorbid behavioral marker. As with loss aversion, the parameter of choice consistency has been implicated in affective disorders yet remains underexamined in experimental research. For example, empirical studies have linked erratic choice behavior to bipolar symptoms (Yechiam, Hayden, Bodkins, O’Donnell, & Hetrick, 2008). Again, however, no studies have considered choice inconsistency as it relates to transdiagnostic risk for internalizing symptoms.
Beyond considering the link between RNT and risk aversion, loss aversion, and choice consistency, a focus on state versus trait questions and transdiagnostic risk may address some of the limitations of prior research. The majority of research on differences in internalizing symptoms and decision-making parameters has tested individuals under neutral affective conditions. However, empirical studies increasingly support the influence of context on decision-making (e.g., Louie, Khaw, & Glimcher, 2013), and a recent review of loss aversion highlights the extent to which loss aversion varies according to environment (Sokol-Hessner & Rutledge, 2019). From a clinical perspective, stress represents a key environmental factor likely to impact decision-making in general (Preston, Buchanan, Stansfield, & Bechara, 2007), and the magnitude of such stress-induced change is linked to individual difference factors such as depression and anxiety symptom severity (Robinson, Bond, & Roiser, 2015). Consequently, incorporating the effects of stress may uncover ways in which decision-making styles, and loss aversion in particular, confer risk for the emergence of internalizing symptoms over time. For instance, it may be that individuals with high cognitive vulnerability but without clinical levels of symptoms exhibit behaviors more akin to clinical samples (e.g., increased loss aversion) not in a trait-like way, but rather when exposed to stressful situations.
Along with the limited focus on trait-like vulnerabilities such as RNT, most studies have assessed decision-making deficits in specific disorders, despite evidence that decision-making abnormalities are transdiagnostic (Gillan, Kosinski, Whelan, Phelps, & Daw, 2016). Moreover, given the high comorbidity of internalizing disorders (Eaton et al., 2013), focusing on a transdiagnostic factor such as RNT as the primary individual difference metric may be more representative of internalizing risk overall. Further, by considering how transdiagnostic RNT after a naturalistic stressor relates to subsequent changes in risk/loss aversion and choice consistency surrounding a laboratory stressor, and by simultaneously measuring stress responses across physiological and affective domains, researchers may elucidate relevant pathways to differences in internalizing symptoms.
Drawing upon hypothesized links between aberrant decision-making under stress and transdiagnostic psychiatric risk, the broad objective of the current study was to prospectively assess whether RNT following a naturalistic stressor predicted downstream responses to an acute laboratory stressor. We recruited a unique sample of young adults who were exposed to Hurricane Irma and reported RNT in its aftermath. Two months later, this sample completed a multimodal, laboratory-based assessment of affect, behavior, and physiology, measured in anticipation of and following a social stressor. Our first specific aim was to determine whether individual differences in post-hurricane RNT were associated with functional impairment following a naturalistic stressor, operationalized as difficulties disengaging from hurricane-related stress upon resuming day-to-day activities. We expected individuals high in RNT to report greater difficulty disengaging from hurricane-related stress upon resuming day-to-day activities (i.e., prolonged response to a naturalistic stressor). Our second aim was to test whether post-hurricane RNT predicted baseline negative affect, cortisol, and decision-making two months later in the laboratory. We hypothesized that higher RNT would predict higher cortisol and negative affect at baseline of the laboratory session. Additionally, in line with research on trait anxiety (Charpentier et al., 2017), we expected that at baseline (under a non-stress condition), post-hurricane RNT would be linked to risk but not loss aversion. Our third aim was to assess whether individual differences in post-hurricane RNT would relate to changes in affect, cortisol, and decision-making in the early-phase response to a stressor (i.e., in anticipation of giving a speech but before actually performing it). Our fourth aim was to assess whether post-hurricane RNT influenced late-phase responses to acute laboratory stress. We expected that individuals high in RNT would exhibit increases in negative affect and cortisol during the early-phase stress response, and that these increases would be sustained during the late-phase stress response. In light of the limited research on decision-making under stress, our hypotheses surrounding decision-making changes in relationship to the laboratory stressor were largely exploratory. However, given the putative influence of environment on loss aversion in particular (Sokol-Hessner & Rutledge, 2019), we anticipated that changes in loss aversion would be displayed during stress exposure.
2. Methods
2.1. Participants
A sample of young adults (N = 90; 73.33% female) participated in partial fulfillment of an undergraduate research requirement, as well as monetary compensation. Participants had a mean age of 18.70 years (SD = 1.39). Nearly half of the sample identified their race as White (48.78%), with 30.00% identifying as Asian or Pacific Islander, 7.78% Black/African American, 6.67% mixed, and 7.78% other. Across all participants, 16.67% identified their ethnicity as Hispanic/Latinx. Fifteen participants in our sample had a PTQ score above 36, which previous studies have cited as the average score for patients with clinical levels of mood and anxiety pathology (Ehring et al., 2011).
2.2. General procedure
This study consisted of two 90–120 min laboratory sessions in Fall of 2017. The initial laboratory session took place an average of 35 days (SD = 5 days; range = 25–42 days) after Hurricane Irma hit Florida in September 2017. The follow-up laboratory session took place an average of 59 days (SD = 6 days; range = 47–70 days) after the initial laboratory session. During the initial session, participants provided written consent and completed a series of self-report measures regarding demographic information, RNT, and hurricane experiences and impairment. During the follow-up session, participants (n = 67) completed questionnaires, the decision-making task, and a stress induction, which was a modified version of the TSST.
The timing and sequence of the follow-up session is depicted in Fig. 1. After completing questionnaires, participants viewed a neutral nature video to establish a baseline state. Participants then moved to a separate room with a different experimenter, who would administer the decision-making task and the stress induction. Participants completed two initial blocks of a decision-making task (namely, the staircase trials and first main task block; see “Decision-making task” section for details). Next, the stress anticipation period began; participants were given the modified TSST instructions (but not their speech topic). Participants then completed a second block of the decision-making task (i.e., the stress anticipation block), after which they received their speech topics. After preparing and delivering their speeches, participants completed a third task block (i.e., the stress recovery block). Following the final task block, participants continued watching the neutral nature video. Mood and cortisol were measured throughout. Throughout the manuscript, we refer to an early-phase and late-phase stress response periods when describing mood, cortisol, and decision-making responses. While these phases map broadly onto the stress anticipation and recovery periods, they are not pure measures of anticipation and recovery because of individual differences in the timing of physiological, affective, and cognitive changes in the context of a stressor (see “Data cleaning and transformation” section for additional details).
Fig. 1.
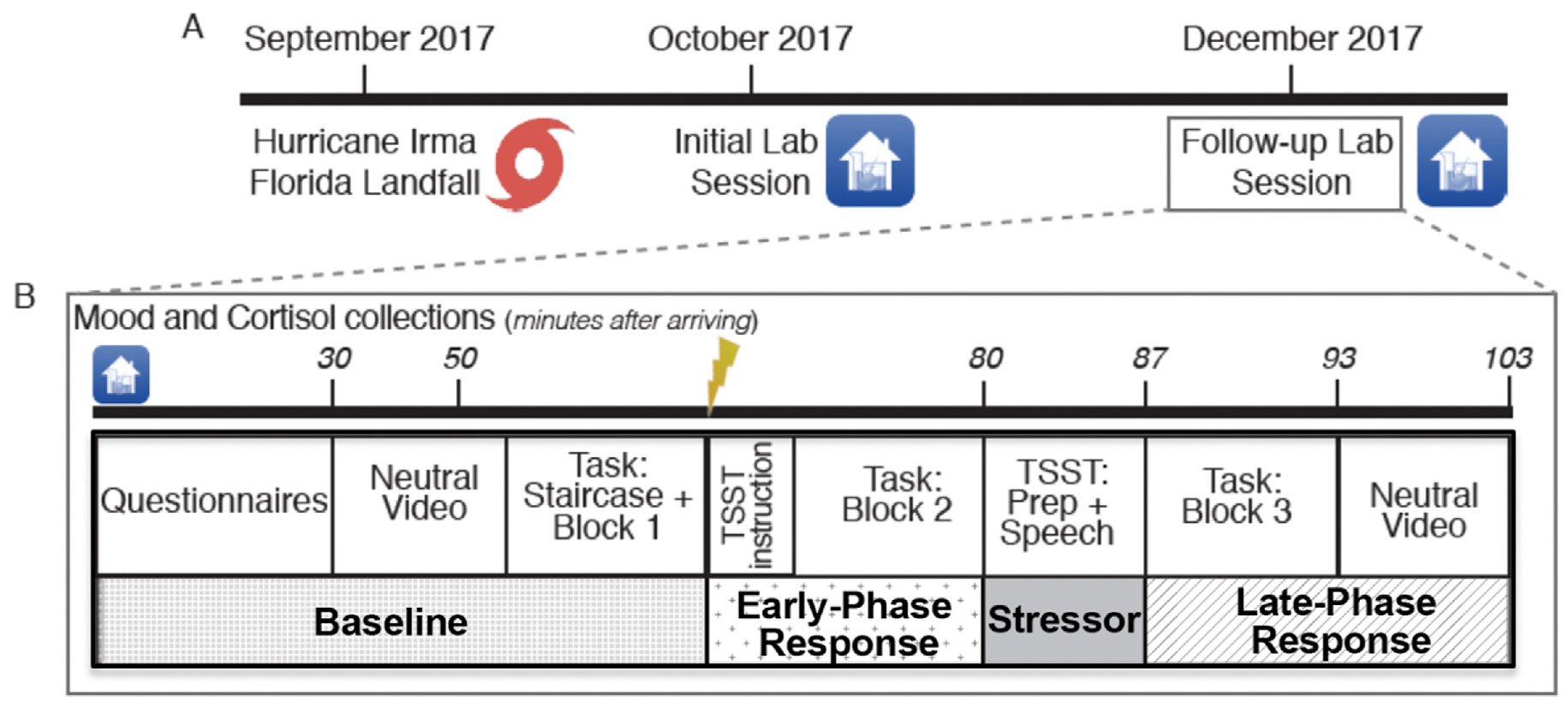
Overview of Study Design. Fig. 1. TSST = modified Trier Social Stress Test. Task = decision-making task. (A) Approximately one month after Hurricane Irma, participants completed an initial laboratory visit during which they rated post-hurricane RNT and hurricane-related impairment. Two months later, participants completed a follow-up laboratory session. (B) During the follow-up laboratory session, participants provided measures of affect, cortisol, and decision making at baseline, as well as in the early-phase (anticipation) and late-phase (recovery) response to an acute social stressor.
2.3. Self-report measures
Hurricane experiences.
Adapted from the Hurricane-Related Traumatic Experiences (La Greca, Silverman, Vernberg, & Prinstein, 1996), the Hurricane Response Questionnaire (HRQ) was used to measure individual differences in hurricane experiences, coping, and stress. The HRQ was administered at the initial laboratory session (Fig. 1). Four HRQ items assessed behavioral impairment following Hurricane Irma: participants rated the extent to which their feelings about their hurricane experience continued to cause impairment in social, occupational, and other domains on a 4-point scale (1 = not at all; 4 = a whole lot). These items were totaled to create an ‘impairment’ score, capturing poor coping and continued disruption of functioning after the hurricane.
Post-hurricane RNT.
We assessed post-hurricane RNT during the initial laboratory session using the 15-item Perseverative Thinking Questionnaire (PTQ; Ehring et al., 2011). Sample items from the PTQ include, “The same thoughts keep going through my mind again and again,” and, “I get stuck on certain issues and cannot move on.” The PTQ exhibits high internal consistency (α = .94-.95) across clinical and non-clinical samples, as well as adequate test-retest reliability (r = 0.69) as measured across a four week period (Ehring et al., 2011).
Negative affect.
We assessed momentary negative affect throughout the follow-up laboratory session using the Negative Affect subscale of the Positive and Negative Affect Schedule (PANAS; Watson, Clark, & Tellegen, 1988). For each of these 10 items, participants rated the extent to which they were currently experiencing each emotion on a 7-point scale (1 = not at all; 7 = very much). A total Negative Affect score was computed by summing the 10 items from this subscale.
Cortisol questionnaire.
Prior to collecting cortisol samples during the follow-up laboratory session (Fig. 1), we administered a questionnaire to gather information about common factors that influence cortisol, including sleep, caffeine intake, smoking patterns, medication use, and menstrual cycle phase (Kirschbaum, Kudielka, Gaab, Schommer, & Hellhammer, 1999).
2.4. Decision-making task
Task description.
Participants completed a behavioral economic task that quantified risk and loss aversion, adapted from a recent study (Charpentier et al., 2017). For each trial, participants made choices between a gamble (always 50% chance between two monetary amounts) and a sure option. The task contained two trials types. “Gain-only” trials involved just potential gains, whereas “Mixed” trials involved both potential gains and losses (Fig. 2). In gain-only trials, participants made a choice between a sure option and a gamble for a potential gain; the amounts for the sure option and gamble both varied across trials. In mixed trials, the sure option was always $0; the gamble presented a 50% chance of winning and 50% chance of losing, with gain and loss values varying on each trial. While only risk aversion influences safe choices on gain-only trials, both risk and loss aversion could contribute to safe choices on mixed gambles. This task thus allowed us to isolate parameters of risk and loss aversion processes. The task also measured choice consistency.
Fig. 2.
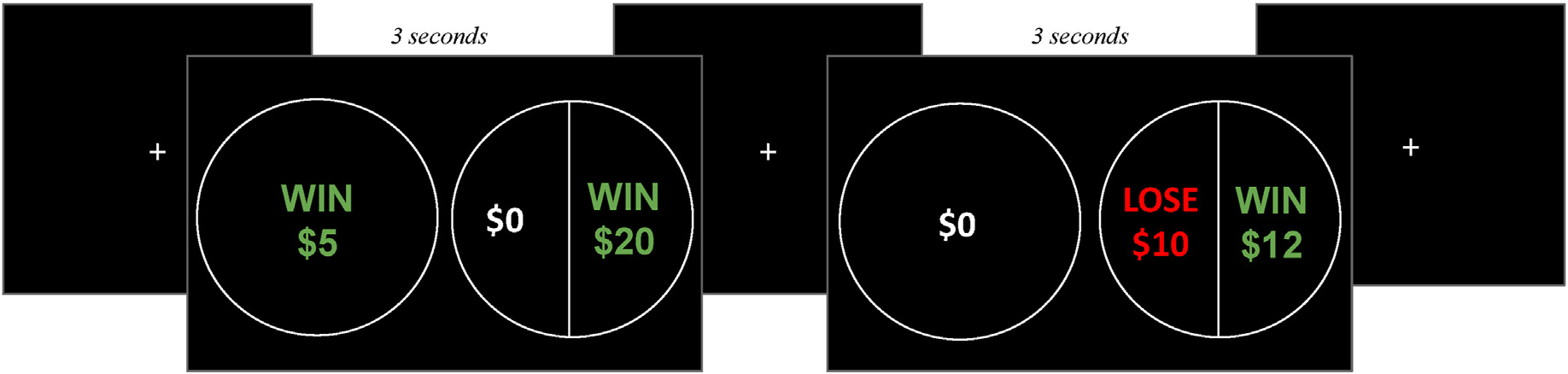
Decision-Making Task Design. Fig. 2. Participants had 3 s to respond to each trial. Two trials types were randomly interleaved: gain only trials (left), involved a certain gain and a 50–50 gain gamble, and mixed trials (right) involved a certain $0 and a 50–50 gain or loss gamble. A fixation cross was shown in between trials.
Task procedure overview.
Participants completed an initial 80 trials implemented in a staircase procedure. The goal of the staircase phase was to establish each participant’s indifference points for both the mixed and gain-only trial types. Indifference points were defined as the difference in expected value between the gamble and the sure option such that a participant was indifferent between the two options. Using these indifference points, an idiographic trial matrix was created for each subject and employed during each of the main task blocks, with trial order randomized within each block. The main task block contained 100 trials and was repeated once at baseline, once during the early-phase stress response period (i.e., anticipation), and once during the late-phase stress response period (i.e., recovery), for a total of three times. Additional details of the task procedure and trial types can be found in the Supplementary Materials.
Task parameters.
To estimate loss aversion, risk aversion, and choice consistency for each participant, a 3-parameter Prospect Theory-derived model was used (Charpentier et al., 2017; Kahneman & Tversky, 2013, pp. 99–127; Sokol-Hessner et al., 2009; Tversky & Kahneman, 1992). For each trial, the subjective utilities (u) of the gamble and sure option were estimated using the following equations, with losses coded as negative values:
| (1) |
| (2) |
In the above equations, λ represents loss aversion; λ > 1 indicates overweighing of losses relative to gains and λ < 1 the converse. ρ represents the curvature of the utility function, which reflects varying sensitivity to changes in values as value increases. If ρ < 1, the utility function is concave for gains and convex for losses, resulting in risk aversion (i.e., greater utility for a sure gain than for a 50/50 gamble with the same expected value); ρ > 1 indicates risk-seeking.
Subjective utility values were passed through a softmax function to estimate the probability of choosing the gamble on each trial (coded as 1 for choosing the gamble and 0 for choosing the sure option), with the inverse temperature parameter μ representing choice consistency:
| (3) |
The model contained 9 parameters in total, with λ, ρ, and μ estimated separately for each condition of the study (baseline, early-phase stress response, and late-phase stress response). Best-fitting parameters were estimated using a maximum likelihood estimation procedure (fminunc function in MATLAB). Reliability was strong for both mixed gamble (α = 0.84) and gain-only (α = 0.81) trials.
2.5. Stress induction
To measure individual differences in responses to acute stress, we administered a stress induction, involving a modified version of the TSST (Kirschbaum et al., 1993). At the beginning of the follow-up laboratory session, participants completed a questionnaire in which they rated a series of topics (e.g., conservation of wetlands in Florida; term limits for federal judges) according to their knowledge level about each topic. Unbeknownst to the participant, their lowest rated topic would be “randomly” selected as their speech topic later in the session. Just prior to giving the speech, participants were told that their speech would be evaluated in real time by the experimenter and videotaped for subsequent evaluation by a committee of raters for content, delivery, strength of argument, eloquence, and sophistication of word choice. Participants were given the topic of their speech and 2 min to prepare. Then, participants delivered their speech for 5 min in front of a video camera and a neutral, cold experimenter.
2.6. Biological samples
Salivary cortisol was sampled in order to measure baseline levels and acute changes in cortisol in response to the stressor (Kirschbaum et al., 1993). Saliva samples were collected in salivettes using a cotton swab and standard procedures (Sarstedt, Germany). Baseline samples were collected approximately 30 and 50 min into the study. Additional samples were taken immediately before and after the speech, as well as 6 and 16 min following the speech (Fig. 1). This sampling procedure allowed us to capture both early and late-phase responses to the speech stressor. All samples were collected between 1 and 6 p.m. and stored in a secure freezer at −20 °F until analysis. Assays were conducted at the Diabetes Research Institute Biomarker and Immunoassay Core Laboratory at the University of Miami. At the conclusion of data collection, samples were thawed, vortexed, and centrifuged at 1500 rpm for 15 min prior to being assayed using the Salimetrics high sensitivity ELISA kit (State College, PA).
2.7. Data analytic plan
Data sharing.
All code and analyses, as well as deidentified data, are publicly available at https://github.com/cbstamatis/RNT.
Data cleaning and transformation.
As previously described, gambling task parameters were estimated using MATLAB. All subsequent data cleaning and analyses were conducted in R. The distributions of all decision-making task parameters were positively skewed (skewness = 1.64 to 4.67), except for ρ in the early-phase stress response block (skewness = 0.43). Consequently, all decision-making task parameters were log-transformed prior to running statistical tests. We were unable to estimate decision-making task parameters for 5 individuals due to problems with estimation and subsequent task crashing (potentially due to erratic choice behavior) for one or more task blocks. In line with prior research using this task (Charpentier et al., 2017), the gambling data for 2 additional participants were excluded because of extremely low values of μ (< 0.02), again indicating random behavior. Due to this missing data, the final number of participants differed somewhat across analyses (cortisol and negative affect: n = 63; risk aversion, loss aversion, and choice consistency: n = 56).
Six cortisol measures and negative affect ratings were collected across the course of the follow-up laboratory session, with two representing baseline, two representing early-phase stress response, and two representing late-phase stress response. To account for individual differences in latency to peak cortisol response surrounding a stressor, we identified each individual’s lowest baseline, highest peak, and lowest recovery cortisol values to be used in analyses. This procedure was also used to determine each individual’s lowest baseline negative affect rating, highest negative affect during stress, and lowest negative affect rating in recovery. The cortisol and negative affect distributions were normally distributed (skewness < 1.5) and therefore were not transformed in analyses. All analyses involving cortisol included gender, caffeine intake, and number of cigarettes as covariates, in line with prior investigations (Kirschbaum et al., 1999). Current use of oral contraceptive medications, current use of other medications, and current menstruation were not associated with cortisol in this sample and were therefore not included as covariates for models with cortisol as an outcome. The intra- and inter-assay coefficients of variation for cortisol determinations were 1.3% and 3.7%, respectively.
Preliminary analyses (stress manipulation check).
We used a series of pairwise linear contrasts to test for changes in the primary outcomes of interest (cortisol, negative affect, and decision-making parameters) between the baseline, early-phase stress response, and late-phase stress response periods.
Primary analyses.
To assess the relationship between RNT and functional impairment following Hurricane Irma (Aim 1), we conducted a linear regression of the post-hurricane impairment in functioning score from the HRQ on PTQ scores following the hurricane. For Aim 2, we conducted separate linear regression models using post-hurricane PTQ scores as predictors of baseline negative affect, cortisol and decision-making tendencies at the follow-up laboratory session. For Aim 3, we tested a series of linear regression models to assess whether PTQ predicted cortisol, affect, and decision-making parameters during the early-phase response to the laboratory stressor. Specifically, in separate models, post-hurricane PTQ was entered as a predictor of cortisol, negative affect, risk aversion, loss aversion, or choice consistency during the early-phase stress response. We then re-ran these models, controlling for respective baseline values of the outcomes of interest (e.g., the effect of PTQ on cortisol during the early-phase response, controlling for baseline cortisol). For Aim 4, we repeated a similar analytic procedure to assess PTQ as a predictor of cortisol, negative affect, risk aversion, loss aversion, and choice consistency during the late-phase stress response. After separately testing the effect of PTQ on each of these outcomes during the late-phase stress response, we re-ran the five models controlling for baseline values of the outcome of interest, then again controlling for the respective outcome of interest during the early-phase stress response. For each analysis, we conducted boostrapping (1000 samples) to obtain unbiased estimates of confidence intervals surrounding our effect. Boostrapped confidence intervals not containing zero provides stronger evidence that an effect is significantly different from zero. These confidence intervals are indicated with CIboot in the results section. Additionally, we present p-values adjusted for multiple comparisons using Benjamini-Hochberg procedures (indicated as padj in the results section).
3. Results
3.1. Stress manipulation check
Results from preliminary analyses supported the effectiveness of the stress task in producing affective and physiological changes (Table 1; Fig. 3). There were significant increases from baseline to the early-phase stress response period for cortisol (M = 0.92, 95% CI: [0.24, 1.60]; t (62) = 2.72, p = .008; d = 0.34) and negative affect (M = 4.51 [3.54, 5.59]; t (62) = 8.36, p < .001; d = 1.05), and significant decreases in these variables from the early to late phase of the stress response (cortisol: M = −2.40 [−2.86; −1.85]; t (62) = −8.62, p < .001; d = −1.09; negative affect: M = −6.06 [−7.32, −4.81]; t (62) = −9.65, p < .001; d = −1.22). The cortisol and negative affect measures during the late-phase stress response period were also significantly lower than their respective baseline values (cortisol: M = −1.48 [−2.32, −0.64]; t (62) = −3.51, p < .001; d −0.44; negative affect: M = −1.53 [−2.40, −0.67]; t (62) = −3.54, p < .001; d = −0.44). For the decision-making task parameters, we observed significant increases in risk aversion (i.e., decreases in risk taking) from baseline to the post-speech (i.e., late-phase stress response) block (M = −0.07 [−0.11, −0.02]; t (55) = −2.78, p = .007; d = −0.37) and increases in choice consistency (M = 0.16 [0.01, 0.31]; t (55) = 2.20, p = .03; d = 0.29) from baseline to the post-speech block. There was also a trending decrease in risk taking (M = −0.04 [−0.09, 0.00]; t (55) = −1.88, p = .07; d = −0.25) from baseline to the pre-speech (i.e., early-phase stress response) block. There were no other significant changes observed in the decision-making task parameters across time (all ps > .10).
Table 1.
Preliminary results (stress manipulation check).
| Outcome | Baseline vs. Early-Phase | Baseline vs. Late-Phase | Early-Phase vs. Late-Phase |
|---|---|---|---|
|
| |||
| Cortisol | t (62) = 2.72, p = .008; M = 0.92 [.24, 1.60]; d = .34 | t (62) = −3.51, p < .001; M = −1.48 [−2.32, −.64]; d = −.44 | t (62) = −8.62, p < .001; M = −2.40 [−2.86; −1.85]; d = −1.09 |
| Negative Affect | t (62) = 8.36, p < .001; M = 4.51 [3.54, 5.59]; d = 1.05 | t (62) = −3.54, p < .001; M = −1.53 [−2.40, −.67]; d = −.44 | t (62) = 9.65, p < .001; M = −6.06 [−7.32, −4.81]; d = −1.22 |
| ρ | t (55) = −1.88, p = .07; M = −.04[−.09, .00]; d = −.25 | t (55) = −2.78, p = .007; M = −.07, [−.11, −.02]; d = −.37 | t (55) = −.90, p = .37; M = −.02 [−.07, .03]; d = −.12 |
| λ | t (55) = 1.10, p = .27; M = .07 [−.06, .19]; d = .15 | t (55) = 1.27, p = .21; M = .08 [−.04, .20]; d = .17 | t (55) = .11, p = .91; M = .01 [−.12, .13]; d = .02 |
| μ | t (55) = .56, p = .58; M = .05 [−.12, .21]; d = .08 | t (55) = 2.20, p = .03; M = .16 [.01, .31]; d = .29 | t (55) = 1.33, p = .19; M = .12 [−.06, .29]; d = .18 |
Note: ρ = risk aversion. λ = loss aversion. μ = choice consistency. Early-phase refers to the early-phase stress response period (anticipation), and late-phase refers to the late-phase stress response period (recovery). M = mean difference; numbers in brackets following mean difference indicate 95% confidence interval around the mean difference. D = Cohen’s d, indicating effect size.
Fig. 3.
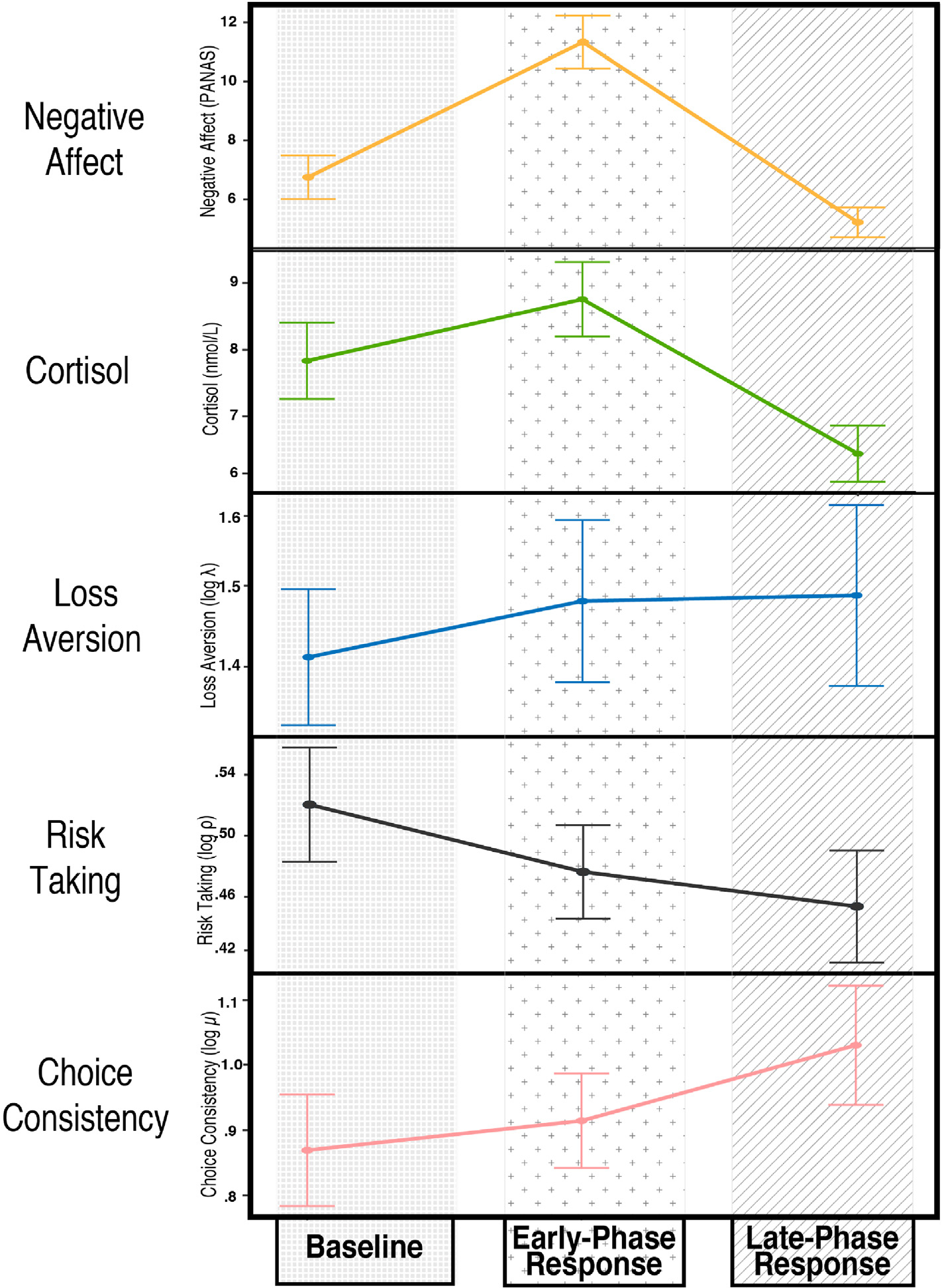
Changes in Affective, Physiological, and Behavioral Measures in Response to Stress. Fig. 3. Average values of the five outcomes of interest are plotted over the course of the laboratory session, in relation to the TSST. The ρ parameter is described here as risk taking to reflect that the observed decrease represents a decreased willingness to take risks, or increased risk aversion.
3.2. Association between RNT and hurricane stress
Participants scoring high on the PTQ one month after Hurricane Irma reported greater functional impairment due to hurricane-related stress. Specifically, PTQ was associated with self-reports of continued day-to-day impairment in functioning due to the stress of Hurricane Irma and evacuation experiences (β = 0.26 [0.05, 0.46], p = .015; rp2 = 0.066; CIboot: [0.08, 0.45]; padj = .044).
3.3. RNT as a predictor of stress-related changes in negative affect
Post-hurricane PTQ predicted greater negative affect (β = 0.47 [0.24, 0.70], p < .001; rp2 = 0.207; CIboot [0.27, 0.77]; padj = .001; Table 2): at the beginning of the follow-up laboratory session (i.e., prior to the stressor) two months after the initial laboratory visit. Higher post-hurricane PTQ was also linked with greater affective changes surrounding the stressor, with greater PTQ predicting increased levels of negative affect during the early-phase stress response (β = .59 [0.37, 0.81], p < .001; rp2 = 0.319; CIboot: [0.37, 0.82]; padj < .001; Table 3). This association remained significant even when baseline negative affect was included in the model (β = 0.27 [0.10, 0.44], p = .001; rp2 = 0.054; CIboot: [0.04, 0.44]; padj = .008). Similarly, we found that greater post-hurricane PTQ predicted higher levels of negative affect during the late-phase response to the stressor (i.e., slower recovery of negative affect; β = 0.50 [0.27, 0.73], p < .001; rp2 = 0.238; CIboot: [0.26, 0.80]; padj < .001; Table 4). However, this association became trending when baseline negative affect was included in the model (β = 0.15 [−0.02, 0.31], p = .077; rp2 = 0.017; CIboot: [−0.01, 0.35]; padj = .125), and non-significant when early-phase negative affect response was included in the model (β = 0.10 [−0.12, 0.33], p = .360; rp2 = 0.007; CIboot: [−0.09, 0.34]; padj = .425).
Table 2.
Effects of post-hurricane PTQ on cortisol, negative affect, and decision-making measured at baseline of a lab visit two months after hurricane exposure.
| Outcome (Baseline) | PTQ effect β [95% CI] |
|---|---|
|
| |
| Cortisol | .19 [−.04, .42]; rp2 = .034 |
| Negative Affect | .47** [.24, .70]; rp2 = .207 |
| ρ | .09 [−.19, .37]; rp2 = .008 |
| λ | −.28* [−.55, −.00]; rp2 = .070 |
| μ | .18 [−.10, .46]; rp2 = .030 |
Note:
p < .10
p < .05
p < .01
p < .001.
ρ = risk aversion. λ = loss aversion. μ = choice consistency. All outcome variables were measured at baseline. ρ, λ, and μ were log-transformed in analyses. Rp2 = squared semi-partial correlation, indicating effect size.
Table 3.
Effects of post-hurricane PTQ on cortisol, negative affect, and decision-making during the early-phase response to an experimental laboratory stressor two months later.
| Outcome (Early-Phase Response) | Model | PTQ effect: β [95% CI]; rp2 | Baseline effect: β [95% CI]; rp2 |
|---|---|---|---|
|
| |||
| Cortisol | (1) | 23* [.02, .45]; rp2 = .051 | – |
| (2) | .10 [−.05, .24]; rp2 = .008 | .72*** [.57, .88]; rp2 = .380 | |
| Negative Affect | (3) | .59** [.37, .81]; rp2 = .319 | – |
| (4) | .27** [.10, .44]; rp2 = .054 | .68*** [.52, .84]; rp2 = .375 | |
| ρ | (5) | .01 [−.27, .29]; rp2 = .000 | – |
| (6) | −.06 [−.24, .12]; rp2 = .003 | .79*** [.61, .96]; rp2 = .613 | |
| λ | (7) | .06 [−.22, .35]; rp2 = .004 | – |
| (8) | .30*** [.14, .47]; rp2 = .079 | .86*** [.70, 1.02]; rp2 = .687 | |
| μ | (9) | −.14 [−.42, .15]; rp2 = .017 | – |
| (10) | −.23† [−.48, .01]; rp2 = .048 | .53*** [.30, .77]; rp2 = .276 | |
Note:
p < .10
p < .05
p < .01
p < .001.
PTQ = post-hurricane Perseverative Thinking Questionnaire. ρ = risk aversion during early-phase stress response. λ = loss aversion during early-phase stress response. μ = choice consistency during early-phase stress response. ρ, λ, and μ were log-transformed in analyses. (1) PTQ effect on peak cortisol during early-phase stress response; (2) PTQ effect on peak cortisol during early-phase stress response controlling for baseline cortisol. (3) PTQ effect on peak negative affect during early-phase stress response; (4) PTQ effect on peak negative affect during early-phase stress response controlling for baseline negative affect; (5) PTQ effect on risk aversion during early-phase stress response; (6) PTQ effect on risk aversion during early-phase stress response controlling for baseline risk aversion; (7) PTQ effect on loss aversion during early-phase stress response; (8) PTQ effect on loss aversion during early-phase stress response controlling for baseline loss aversion; (9) PTQ effect on choice consistency during early-phase stress response; (6) PTQ effect on choice consistency during early-phase stress response controlling for baseline choice consistency. Rp2 = squared semi-partial correlation, indicating effect size.
Table 4.
Effects of post-hurricane PTQ on cortisol, negative affect, and decision-making during the late-phase response to an experimental laboratory stressor two months later.
| Outcome (Late-Phase Response) | Model | PTQ effect: β [95% CI]; rp2 | Baseline effect: β [95% CI]; rp2 | Anticipation effect: β [95% CI]; rp2 |
|---|---|---|---|---|
|
| ||||
| Cortisol | (1) | .32** [.10, .54]; rp2 = .095 | – | – |
| (2) | .22* [.04, .39]; rp2 = .043 | .55*** [.35, .75]; rp2 = .226 | – | |
| (3) | .13* [.01, .26]; rp2 = .014 | – | .82*** [.67, .97]; rp2 = .437 | |
| Negative Affect | (4) | .50*** [.27, .73]; rp2 = .238 | – | – |
| (5) | .15† [−.02, .31]; rp2 = .017 | .75*** [.59, .91]; rp2 = .451 | – | |
| (6) | .10 [−.12, .33]; rp2 = .007 | – | .68*** [.47, .89]; rp2 = .312 | |
| ρ | (7) | .18 [−.10, .46]; rp2 = .023 | – | – |
| (8) | .11 [−.07, .28]; rp2 = .010 | .78*** [.62, .95]; rp2 = .607 | – | |
| (9) | .17† [−.02, .36]; rp2 = .026 | – | .73*** [.55, .92]; rp2 = .540 | |
| λ | (10) | .02 [−.27, .30]; rp2 = .000 | – | – |
| (11) | .27*** [.13, .41]; rp2 = .062 | .92*** [.79, 1.05]; rp2 = .784 | – | |
| (12) | −.04 [−.20, .12]; rp2 = .001 | – | .83*** [.68, .99]; rp2 = .690 | |
| μ | (13) | −.03 [−.32, .25]; rp2 = .001 | – | – |
| (14) | −.16 [−.37, .05]; rp2 = .023 | .98*** [.48, .89]; rp2 = .454 | – | |
| (15) | −.02 [−.23, .28]; rp2 = .001 | – | .46*** [.21, .70]; rp2 = .208 | |
Note:
p < .10
p < .05
p < .01
p < .001.
PTQ = post-hurricane Perseverative Thinking Questionnaire. ρ = risk aversion during late-phase stress response. λ = loss aversion during late-phase stress response. μ = choice consistency during late-phase stress response. ρ, λ, and μ were log-transformed in analyses. (1) PTQ effect on lowest cortisol during late-phase stress response; (2) PTQ effect on lowest cortisol during late-phase stress response controlling for baseline cortisol; (3) PTQ effect on lowest cortisol during late-phase stress response controlling for cortisol during peak early-phase stress response. (4) PTQ effect on lowest negative affect during late-phase stress response; (5) PTQ effect on lowest negative affect during late-phase stress response controlling for baseline negative affect; (6) PTQ effect on lowest negative affect during late-phase stress response controlling for negative affect during peak early-phase stress response; (7) PTQ effect on risk aversion during late-phase stress response stress; (8) PTQ effect on risk aversion during late-phase stress response controlling for baseline risk aversion; (9) PTQ effect on risk aversion during late-phase stress response controlling for risk aversion during early-phase stress response; (10) PTQ effect on loss aversion during late-phase stress response; (11) PTQ effect on loss aversion during late-phase stress response stress controlling for baseline loss aversion; (12) PTQ effect on loss aversion during late-phase stress response stress controlling for loss aversion during early-phase stress response; (13) PTQ effect on choice consistency during late-phase stress response; (14) PTQ effect on choice consistency during late-phase stress response controlling for baseline choice consistency. (15) PTQ effect on choice consistency during late-phase stress response controlling for choice consistency during early-phase stress response. Rp2 = squared semi-partial correlation, indicating effect size.
3.4. RNT as a predictor of stress-related changes in cortisol
Post-hurricane PTQ was not a significant predictor of baseline cortisol levels (β = 0.19 [−0.04, 0.42], p = .106; rp2 = 0.034; CIboot: [−0.08, 0.43]; padj = .159) measured at the start of the second lab visit. However, during the stress manipulation, participants scoring higher on the PTQ after the hurricane exhibited increased cortisol during the early-phase stress response period (β = 0.23 [0.02, 0.45]; p = .034; rp2 = 0.051; CIboot: [0.00, 0.49]; padj = .084). This association became nonsignificant when baseline cortisol was included in the model (β = 0.10 [−0.05, 0.24], p = .179; rp2 = 0.008; CIboot: [−0.04, 0.25]; padj = .252). Stronger effects were observed with regard to PTQ and cortisol levels during the late-phase stress response period. Specifically, post-hurricane PTQ scores predicted greater levels of cortisol in the late-phase response to the lab-based stressor (β = 0.32 [0.10, 0.54], p = .005; rp2 = 0.095; CIboot: [0.10, 0.60]; padj = .018), an association that remained significant when controlling for cortisol during the baseline (β = 0.22 [0.04, 0.39], p = .018; rp2 = 0.043; CIboot: [0.07, 0.44]; padj = .049) and early-phase response (β = 0.13 [0.01, 0.26]; p = .045; rp2 = 0.014; CIboot: [0.05, 0.23]; padj = .097) periods.
3.5. RNT as a predictor of stress-related changes in decision-making
Post-hurricane PTQ predicted lower levels of loss-averse decision making (β = −0.27 [−0.55, 0.00], p = .049; rp2 = 0.070; Fig. 4) at the start of the lab session, though this effect became trending when bootstrapped sampling was used to estimate confidence intervals (CIboot: [−0.78, −0.02]; padj = .097). In contrast, there was no association between PTQ and baseline risk aversion (β = 0.09 [−0.19, 0.37], p = .521; rp2 = 0.008; CIboot: [−0.14, 0.33]; padj = .568) or choice consistency (β = 0.18 [−0.10, 0.46], p = .205; rp2 = 0.030; CIboot: [−0.09, 0.50]; padj = .267). During the early-phase response to the stressor, post-hurricane PTQ predicted greater loss aversion (β = 0.30 [0.14, 0.47], p < .001; rp2 = 0.079; CIboot: [0.17, 0.52]; padj = .003), controlling for the influence of baseline loss aversion. Post-hurricane PTQ was also marginally associated with lower choice consistency in the early-phase stress response period (β = −0.23† [−0.48, 0.01], p = .065; rp2 = 0.048; CIboot: [−0.49, −0.05]; padj = .117), accounting for baseline choice consistency. Again, the relationship between PTQ and risk aversion was nonsignificant during the early-phase stress response period (Table 3). However, post-hurricane PTQ predicted marginally lower risk aversion (i.e., greater risk taking) during the late-phase response to stress when controlling for risk aversion during the early-phase stress response period (β = 0.17 [−0.02, 0.36], p = .078; rp2 = 0.026; CIboot: [−0.04, 0.43]; padj = .125). PTQ was also associated with greater loss aversion during the late-phase stress response, controlling for baseline loss aversion (β = 0.27 [0.13, 0.41], p < .001; rp2 = 0.062; CIboot: [0.11, 0.48]; padj = .002). There was no relationship of post-hurricane PTQ with choice consistency during the late-phase stress response period.
Fig. 4.
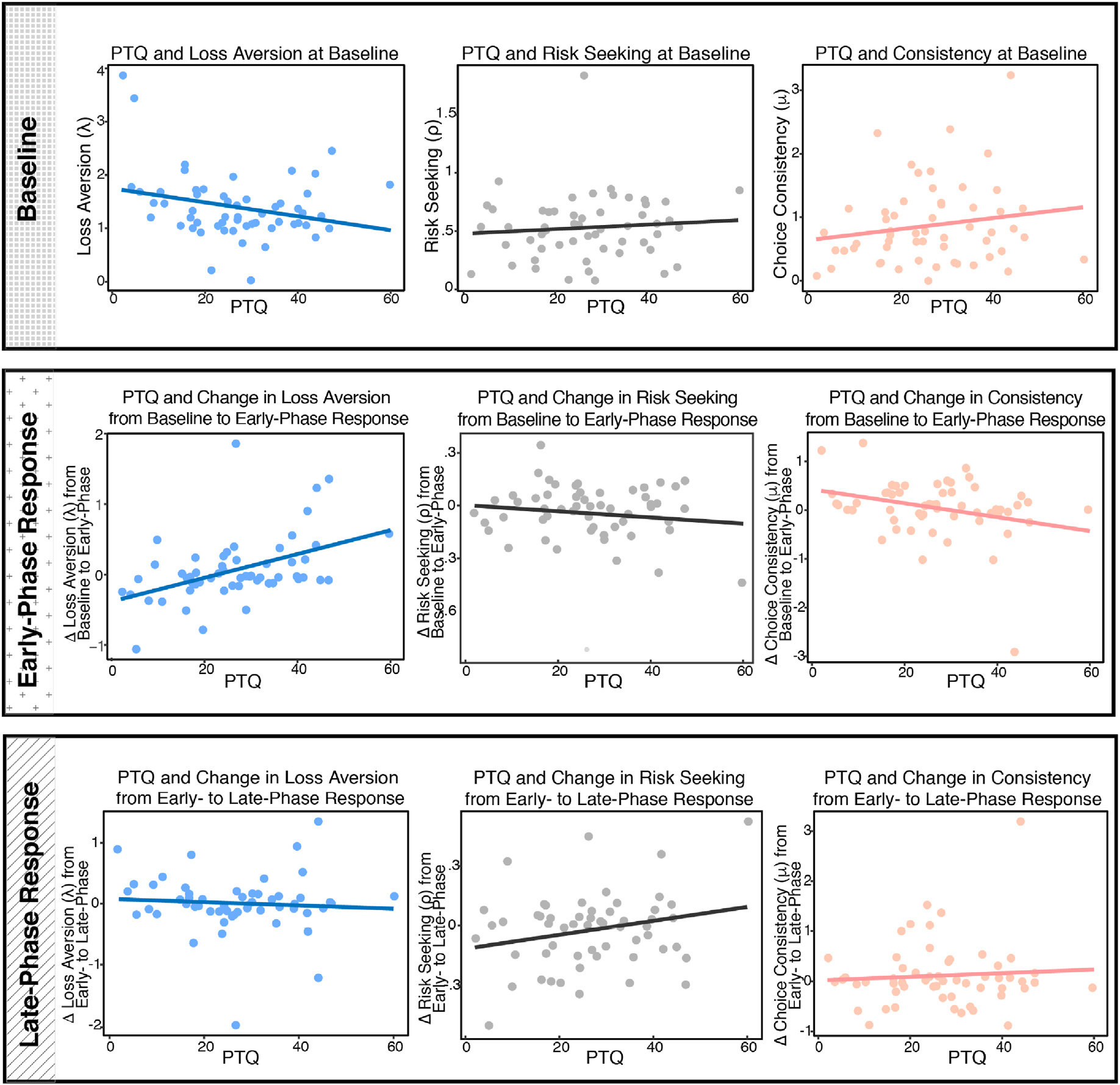
Associations of RNT with the Decision-Making Task Parameters at Baseline, Stress Anticipation, and Stress Recovery. Fig. 4. PTQ = Perseverative Thinking Questionnaire. ρ = risk aversion. λ = loss aversion. μ = choice consistency. λ, and μ were log-transformed in analyses. For ease of visual interpretation, change scores are plotted here from baseline to anticipation (column 2) and from anticipation to recovery (column 3); however, all statistics reported are from regression models described in the text. PTQ was significantly negatively related with λ at baseline (β = −0.27, p < .05). Conversely, PTQ positively predicted increases in loss aversion from baseline to early-phase response (β = 0.30, p < .001), and this increase remained significant during the late-phase response (β = 0.27, p < .001). PTQ was also linked with marginal decreases in choice consistency from baseline to early-phase response (β = −0.23, p < .10). There was a marginal relationship between PTQ and increases in risk taking from the early to late-phase response periods (β = .17, p < .10). There were no other significant associations of PTQ with the decision-making task parameters.
4. Discussion
The current investigation is the first to measure RNT after a major naturalistic stressor, and to evaluate its relationship to downstream stress responding across multiple systems. Results linked post-hurricane impairment with RNT, which, in turn, predicted increases in cortisol, negative affect, and loss aversion in the early-phase response to a laboratory stressor two months later. High-RNT individuals also exhibited heightened negative affect and cortisol during the late-phase response to the stressor. Findings converged on RNT being linked to maladaptive stress responses, highlighting a potential pathway by which RNT may relate to individual differences in risk for affective disorders.
Our results provide an empirical link of transdiagnostic RNT with both naturalistic and laboratory stress responding. In line with diathesis-stress perspectives (Robinson & Alloy, 2003), post-hurricane RNT levels were correlated with poorer functioning. This association dovetails with research on cognitive vulnerabilities and other natural disasters, including worry predicting poorer immune functioning after an earthquake (Segerstrom, Solomon, Kemeny, & Fahey, 1998) and depressive symptoms after Hurricane Katrina (Weems et al., 2007). Our study demonstrates that individual differences in levels of RNT after a real-world stressor also exhibited maladaptive stress responding across multiple systems months later in the controlled setting of the laboratory.
By pairing the modified TSST with a decision-making task, we demonstrated that individual differences in RNT was linked to stress-related alterations in decision-making. Expanding on previous studies primarily focused on risk aversion, we disentangled the effects of stress on both risk and loss aversion (Charpentier et al., 2017). Our results indicated that when facing an impending stressor, individuals with relatively higher RNT exhibited increased loss aversion and more sporadic response patterns. The central role of RNT in decision-making under stress described here may inform mixed findings from previous literature, wherein stress has been associated with increased risk aversion (Porcelli & Delgado, 2009), increased risk-seeking (Stamatis et al., 2020; Starcke, Wolf, Markowitsch, & Brand, 2008), and—in a study that separately estimated risk aversion, loss aversion, and choice consistency (Sokol-Hessner, Raio, Gottesman, Lackovic, & Phelps, 2016)—no changes in decision-making. Interestingly, one study that used the threat of shock to induce stress found no stress-related changes in decision-making (Charpentier, Hindocha et al., 2017), suggesting that the type of stressor may also moderate this effect.
Our findings linking RNT with loss aversion under stress, particularly in the context of concurrent stress-related changes in cortisol and negative affect, build on existing literature to suggest potential individual difference mechanisms. As previously described, we found that during early-phase stress response (encompassing the anticipatory response to a stressor), individuals higher in RNT exhibited greater loss aversion. Past research has posited loss aversion as a neurobiological marker of depression (Chandrasekhar Pammi et al., 2015). However, no previous studies have measured loss aversion in the context of risk for depression and other affective disorders, nor in conjunction with physiological and affective measures during stress. Our results support loss aversion as a potential individual difference marker and, by linking decision-making alterations with corresponding changes in mood and cortisol, point to ways in which stress may exacerbate symptoms via cognitive, affective, and behavioral changes.
Although broad overlapping patterns of RNT and stress responding emerged, we also observed differential relationships between RNT and stress responsivity across measurement modalities and time. Individual differences in RNT predicted greater increases in NA, loss aversion, and choice inconsistency during the early-phase stress response, but only predicted levels of cortisol and negative affect during the late-phase stress response. Previous studies have linked poor cortisol recovery with worry and rumination (Capobianco et al., 2018), and it has been theorized that sustained cognitive representations of the stressor mediates the association between stress exposure and physiological activation (Brosschot, Pieper, & Thayer, 2005). Our results indicate that a single measurement of behavior, affect and physiology may be insufficient to fully capture the impact of stress on individual differences.
Our results should be considered in light of the study’s strengths and limitations, which point to avenues for future research. Hurricane Irma coincided with the start of the fall semester, and as a result we were not able to obtain a true baseline measure of RNT prior to the hurricane, limiting the interpretation of the link between RNT and hurricane-related impairment, as well as the directionality of the association. Given that previous studies suggest that the temporal stability of disorder-specific RNT is quite high (e.g., in rumination; Roberts, Gilboa, & Gotlib, 1998), we cannot determine with certainty whether our results reflect more general effects of RNT, or RNT specifically in the context of hurricane stress. Similarly, although the stress induction produced expected increases in cortisol and negative affect (Kirschbaum et al., 1993), we cannot fully rule out time effects on decision-making alterations. Between subjects designs with larger samples will permit researchers to more clearly parse these effects. An additional goal for future studies is expanding this paradigm to groups beyond our young adult sample. While young adulthood represents a formative time for psychopathology (Beiter et al., 2015), especially after natural disasters (Bianchini et al., 2015), future studies can test whether similar relationships between RNT and decision-making under stress occur in general adults from high-risk and clinical samples. As another limitation, our study did not test relationships between RNT, decision-making, and clinical symptoms. Given that RNT is considered a risk factor for psychopathology (Spinhoven et al., 2018), future studies should model these multimodal, maladaptive stress response patterns as mediators between RNT and later psychopathology development.
The present investigation combined multiple modalities to characterize the association between individual differences in RNT on biological, behavioral, and affective responses to stress. Converging evidence across these systems provided strong support for RNT as a factor that may impact stress-related coping. Differences across levels of analysis in the relationship between RNT and stress responding highlighted the importance of assessing stress reactions from multiple perspectives. These findings lend support for mechanisms by which RNT may act synergistically with stress in the context of diathesis-stress models, an important step in improving predictions of individual differences in affective pathology.
Supplementary Material
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Declaration of competing interest
The authors declare no conflict of interest.
CRediT authorship contribution statement
Caitlin A. Stamatis: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing - review & editing, Writing - original draft. Nikki A. Puccetti: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing - review & editing, Writing - original draft. Caroline J. Charpentier: Data curation, Formal analysis, Writing - original draft. Aaron S. Heller: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing - review & editing. Kiara R. Timpano: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing - review & editing.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.brat.2020.103609.
References
- Allen AP, Kennedy PJ, Cryan JF, Dinan TG, & Clarke G (2014). Biological and psychological markers of stress in humans: Focus on the trier social stress test. Neuroscience & Biobehavioral Reviews, 38, 94–124. [DOI] [PubMed] [Google Scholar]
- Arditte KA, Shaw AM, & Timpano KR (2016). Repetitive negative thinking: A transdiagnostic correlate of affective disorders. Journal of Social and Clinical Psychology, 35(3), 181–201. [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, & Emery G (1979). Cognitive therapy of depression.Guilford Publications. [Google Scholar]
- Beiter R, Nash R, McCrady M, Rhoades D, Linscomb M, Clarahan M, et al. (2015). The prevalence and correlates of depression, anxiety, and stress in a sample of college students. Journal of Affective Disorders, 173, 90–96. [DOI] [PubMed] [Google Scholar]
- Bianchini V, Roncone R, Giusti L, Casacchia M, Cifone MG, & Pollice R (2015). PTSD growth and substance abuse among a college student community: Coping strategies after 2009 L’aquila earthquake. Clinical Practice and Epidemiology in Mental Health: CP & EMH, 11, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SJ, & Gagne C (2018). Anxiety, depression, and decision making: A computational perspective. Annual Review of Neuroscience, 41, 371–388. [DOI] [PubMed] [Google Scholar]
- Brosschot JF, Pieper S, & Thayer JF (2005). Expanding stress theory: Prolonged activation and perseverative cognition. Psychoneuroendocrinology, 30(10), 1043–1049. [DOI] [PubMed] [Google Scholar]
- Calmes CA, & Roberts JE (2007). Repetitive thought and emotional distress: Rumination and worry as prospective predictors of depressive and anxious symptomatology. Cognitive Therapy and Research, 31(3), 343–356. [Google Scholar]
- Capobianco L, Morris JA, & Wells A (2018). Worry and rumination: Do they prolong physiological and affective recovery from stress? Anxiety, Stress & Coping, 31(3), 291–303. [DOI] [PubMed] [Google Scholar]
- Chandrasekhar Pammi VS, Pillai Geethabhavan Rajesh P, Kesavadas C, Rappai Mary P, Seema S, Radhakrishnan A, et al. (2015). Neural loss aversion differences between depression patients and healthy individuals: A functional MRI investigation. The Neuroradiology Journal, 28(2), 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier CJ, Aylward J, Roiser JP, & Robinson OJ (2017). Enhanced risk aversion, but not loss aversion, in unmedicated pathological anxiety. Biological Psychiatry, 81(12), 1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier CJ, Martino BD, Sim AL, Sharot T, & Roiser JP (2015). Emotion-induced loss aversion and striatal-amygdala coupling in low-anxious individuals. Social Cognitive and Affective Neuroscience, 11(4), 569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton NR, Krueger RF, Markon KE, Keyes KM, Skodol AE, Wall M, ... Grant BF (2013). The structure and predictive validity of the internalizing disorders. Journal of Abnormal Psychology, 122(1), 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring T, & Watkins ER (2008). Repetitive negative thinking as a transdiagnostic process. International Journal of Cognitive Therapy, 1(3), 192–205. [Google Scholar]
- Ehring T, Zetsche U, Weidacker K, Wahl K, Schönfeld S, & Ehlers A (2011). The Perseverative Thinking Questionnaire (PTQ): Validation of a content-independent measure of repetitive negative thinking. Journal of Behavior Therapy and Experimental Psychiatry, 42(2), 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann JB, Berns GS, & Dunlop BW (2017). Hyper-responsivity to losses in the anterior insula during economic choice scales with depression severity. Psychological Medicine, 47(16), 2879–2891. [DOI] [PubMed] [Google Scholar]
- Gibb BE, & Coles ME (2005). Cognitive vulnerability-stress models of psychopathology. Development and Psychopathology: A vulnerability-stress perspective,104–135. [Google Scholar]
- Gillan CM, Kosinski M, Whelan R, Phelps EA, & Daw ND (2016). Characterizing a psychiatric symptom dimension related to deficits in goal-directed control. Elife, 5, e11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgetta C, Grecucci A, Zuanon S, Perini L, Balestrieri M, Bonini N, ... Brambilla P (2012). Reduced risk-taking behavior as a trait feature of anxiety. Emotion, 12(6), 1373. [DOI] [PubMed] [Google Scholar]
- Hartley S, Haddock G, Vasconcelos E Sa D, Emsley R, & Barrowclough C (2014). An experience sampling study of worry and rumination in psychosis. Psychological Medicine, 44(8), 1605–1614. [DOI] [PubMed] [Google Scholar]
- Howes OD, McDonald C, Cannon M, Arseneault L, Boydell J, & Murray RM (2004). Pathways to schizophrenia: The impact of environmental factors. Cambridge, UK: Cambridge University Press. [DOI] [PubMed] [Google Scholar]
- Kahneman D, & Tversky A (2013). Prospect theory: An analysis of decision under risk Handbook of the fundamentals of financial decision making: Part I. World Scientific. [Google Scholar]
- Kertz SJ, Stevens KT, & Klein KP (2017). The association between attention control, anxiety, and depression: The indirect effects of repetitive negative thinking and mood recovery. Anxiety, Stress & Coping, 30(4), 456–468. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, & Hellhammer DH (1999). Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus-pituitary-adrenal axis. Psychosomatic Medicine, 61(2), 154–162. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke K-M, & Hellhammer DH (1993). The ‘Trier Social Stress Test’–a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology, 28(1–2), 76–81. [DOI] [PubMed] [Google Scholar]
- La Greca AM, Silverman WK, Vernberg EM, & Prinstein MJ (1996). Symptoms of posttraumatic stress in children after hurricane andrew: A prospective study. Journal of Consulting and Clinical Psychology, 64(4), 712. [DOI] [PubMed] [Google Scholar]
- Louie K, Khaw MW, & Glimcher PW (2013). Normalization is a general neural mechanism for context-dependent decision making. Proceedings of the National Academy of Sciences, 110(15), 6139–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, & Nolen-Hoeksema S (2011). Rumination as a transdiagnostic factor in depression and anxiety. Behaviour Research and Therapy, 49(3), 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, & Lyubomirsky S (2008). Rethinking rumination. Perspectives on Psychological Science, 3(5), 400–424. [DOI] [PubMed] [Google Scholar]
- Porcelli AJ, & Delgado MR (2009). Acute stress modulates risk taking in financial decision making. Psychological Science, 20(3), 278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston SD, Buchanan TW, Stansfield RB, & Bechara A (2007). Effects of anticipatory stress on decision making in a gambling task. Behavioral Neuroscience,121(2), 257. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Gilboa E, & Gotlib IH (1998). Ruminative response style and vulnerability to episodes of dysphoria: Gender, neuroticism, and episode duration. Cognitive Therapy and Research, 22(4), 401–423. [Google Scholar]
- Robinson MS, & Alloy LB (2003). Negative cognitive styles and stress-reactive rumination interact to predict depression: A prospective study. Cognitive Therapy and Research, 27(3), 275–291. [Google Scholar]
- Robinson OJ, Bond RL, & Roiser JP (2015). The impact of stress on financial decision-making varies as a function of depression and anxiety symptoms. PeerJ, 3, e770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstrom SC, Solomon GF, Kemeny ME, & Fahey JL (1998). Relationship of worry to immune sequelae of the Northridge earthquake. Journal of Behavioral Medicine, 21(5), 433–450. [DOI] [PubMed] [Google Scholar]
- Sokol-Hessner P, Hsu M, Curley NG, Delgado MR, Camerer CF, & Phelps EA (2009). Thinking like a trader selectively reduces individuals’ loss aversion. Proceedings of the National Academy of Sciences, 106(13), 5035–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol-Hessner P, Raio CM, Gottesman SP, Lackovic SF, & Phelps EA (2016). Acute stress does not affect risky monetary decision-making. Neurobiology of stress, 5, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol-Hessner P, & Rutledge RB (2011). The psychological and neural basis of loss aversion. Current Directions in Psychological Science, 28(1), 20–27. [Google Scholar]
- Sokol-Hessner P, & Rutledge RB (2019). The psychological and neural basis of loss aversion. Current Directions in Psychological Science, 28(1), 20–27. [Google Scholar]
- Spinhoven P, van Hemert AM, & Penninx BW (2018). Repetitive negative thinking as a predictor of depression and anxiety: A longitudinal cohort study. Journal of Affective Disorders, 241, 216–225. [DOI] [PubMed] [Google Scholar]
- Stamatis CA, Engelmann JB, Ziegler C, Domschke K, Hasler G, & Timpano KR (2020). A neuroeconomic investigation of 5-HTT/5-HT1A gene variation, social anxiety, and risk-taking behavior. Anxiety, Stress & Coping, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcke K, Wolf OT, Markowitsch HJ, & Brand M (2008). Anticipatory stress influences decision making under explicit risk conditions. Behavioral Neuroscience,122(6), 1352. [DOI] [PubMed] [Google Scholar]
- Sutker PB, Corrigan SA, Sundgaard-Riise K, Uddo M, & Allain AN (2002). Exposure to war trauma, war-related PTSD, and psychological impact of subsequent hurricane. Journal of Psychopathology and Behavioral Assessment, 24(1), 25–37. [Google Scholar]
- Tversky A, & Kahneman D (1992). Advances in prospect theory: Cumulative representation of uncertainty. Journal of Risk and Uncertainty, 5(4), 297–323. [Google Scholar]
- Watson D, Clark LA, & Tellegen A (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063. [DOI] [PubMed] [Google Scholar]
- Weems CF, Pina AA, Costa NM, Watts SE, Taylor LK, & Cannon MF (2007). Predisaster trait anxiety and negative affect predict posttraumatic stress in youths after Hurricane Katrina. Journal of Consulting and Clinical Psychology, 75(1), 154. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2017). Depression and other common mental disorders: Global health estimates.
- Yechiam E, Hayden EP, Bodkins M, O’Donnell BF, & Hetrick WP (2008). Decision making in bipolar disorder: A cognitive modeling approach. Psychiatry Research, 161(2), 142–152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


