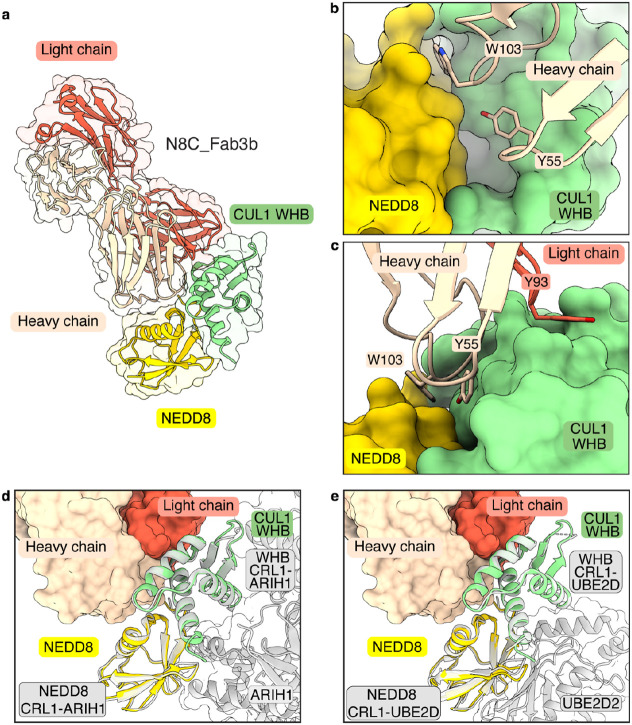
Fig. 3: Crystal structure of N8C_Fab3b in complex with neddylated CUL1WHB reveals capture of the active conformation.
a, Crystal structure of N8C_Fab3b in complex with neddylated CUL1WHB at 2.6 Å resolution. N8C_Fab3b recognizes a unique interface spanning both NEDD8 and CUL1. b, Focused view of the N8C_Fab3b wedge consisting of residues Y55 and W103 of the Fab heavy chain burying itself in the groove between the CUL1 WHB domain and NEDD8. c, The N8C_Fab3b wedge is further stabilized by Y93 hooking into the edge of the CUL1 WHB domain. d, Side-by-side comparison of the NEDD8-CUL1WHB bound by N8C_Fab3b with the one seen in an active CRL1FBXW7-UBE2L3/ARIH1 complex (7B5L) reveals N8C_Fab3b capturing the active conformation of NEDD8-CUL1WHB. Alignments were performed on NEDD8-CUL1WHB. e, Alignment of CUL1WHB-NEDD8 bound by N8C_Fab3b and the one found in the active CRL1BTRC-UBE2D2 complex (6TTU) further demonstrates N8C_Fab recognizing the active NEDD8-CUL1WHB conformation and being compatible with ubiquitin carrying enzymes. Alignments were performed on NEDD8-CUL1WHB.