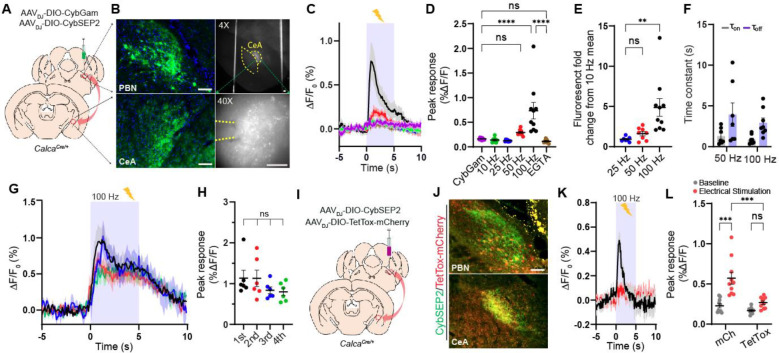
Figure 2. Imaging the LDCV release in brain slices.
(A and B) Schematic and representative images showing CybSEP2 targeted region, its projection to the CeA and expression of CybSEP2 in the PBN and the CeA of the CalcaCre/+ brain slices. Images in the right panel (B) show slices containing the CeA for slice imaging experiment (Scale bar, 100 μm).
(C and D) Average traces of fluorescence change in response to various electrical stimulation and quantification of the data (C). For extracellular calcium removal, 5 mM EGTA was used instead of CaCl2. Each trace is the average of 7–9 trials in 24 slice slices prepared from 4 mice (****p < 0.0001 via one-way ANOVA followed by Šidák multiple comparisons).
(E) Quantification of fold change from percent ΔF/F0 peak intensity compared to 10 Hz in (D) (**p < 0.01 via one-way ANOVA followed by Tukey’s multiple comparisons).
(F) Time constant (τ) of CybSEP2 expressing neurons during electrical stimulation at 50 Hz (n=7) and 100 Hz (n=8). The rising (τon) and decay (τoff) phases were determined by fitting across an entire stimulation period (τon = 1.30 ± 0.37, τoff = 3.93 ± 1.44 at 50 Hz; τon = 0.85 ± 0.18, τoff = 2.93 ± 0.55 at 100 Hz).
(G and H) Average traces of fluorescence change in response to repeated electrical stimulation at 100 Hz and quantification of percent ΔF/F0 peak intensity in (G). Each trial was measured at 5 min interval between trials (n=6; ns, not significant via one-way ANOVA followed by Tukey’s multiple comparisons).
(I and J) Schematic brain region targeted for viral injection and co-expression of CybSEP2 and TetTox-mCherry in the PBN and in the CeA of CalcaCre/+ (Scale bar, 100 μm).
(K and L) The trace of fluorescence change in CybSEP2 with mCherry (n=10 slices from 3 mice) or TetTox-mCherry (n=10 slices from 3 mice) expressing neurons during electrical stimulation at 100 Hz and quantification of date in (K) (***p < 0.0001 via two-way ANOVA followed by Šidák multiple comparisons). Data are represented as mean ± SEM.