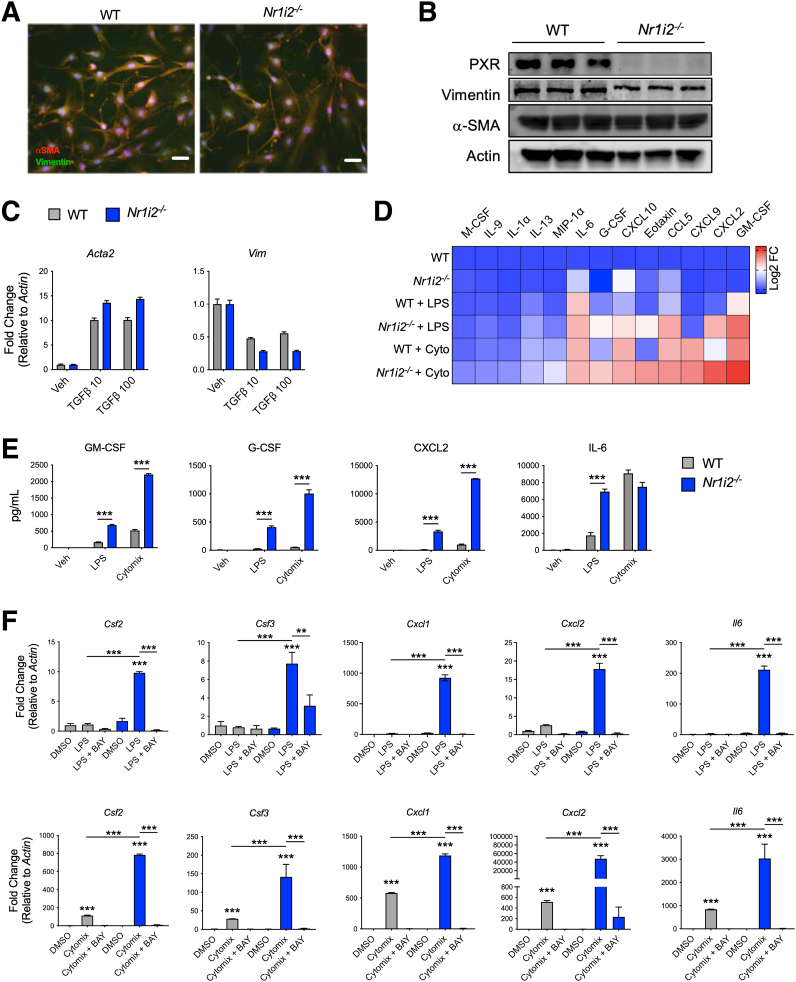
Figure 8.
Loss of intestinal PXR signaling imparts increased inflammatory reactivity in intestinal myofibroblasts. (A) Staining of αSMA and vimentin in primary myofibroblasts isolated from the colon of WT(Nr1i2+/+) and Nr1i2-/- mice to show fibroblastic morphology (scale bar = 250 μm). (B) Western blots demonstrating protein levels of PXR, αSMA, and vimentin in myofibroblasts isolated from the colon of WT(Nr1i2+/+) and Nr1i2-/- mice. (C) Responsiveness of colonic myofibroblasts from WT(Nr1i2+/+) and Nr1i2-/- mice to TGFβ (reduced serum conditions for 24 hours and then stimulated with TGFβ1 for 24 hours) measured by mRNA expression for Acta2 (αSMA) and Vim (n = 3 per group). (D) Heatmap showing the most robustly up-regulated cytokines in cell supernatants from colonic myofibroblastss isolated from WT(Nr1i2+/+) and Nr1i2-/- mice after stimulation with LPS or cytomix (TNFα, IL1β, and IFNγ) for 24 hours (each tile is the average of 4 replicates). (E) Quantification of total cytokine levels in respective myofibroblasts groups after 24 hours of stimulation with cytomix or LPS (n = 4 per group). (F) Gene expression of different cytokines in colonic myofibroblasts isolated from WT(Nr1i2+/+) and Nr1i2-/- mice and pretreated with vehicle (DMSO) or the NF-κB kinase inhibitor BAY 11-7082 (BAY) for 4 hours and then stimulated with LPS or cytomix for additional 12 hours (n = 3 per group). Data are presented as mean ± standard error of the mean. One-way analysis of variance with Tukey post hoc test. ∗P < .05, ∗∗P < .01, ∗∗∗P < .001.