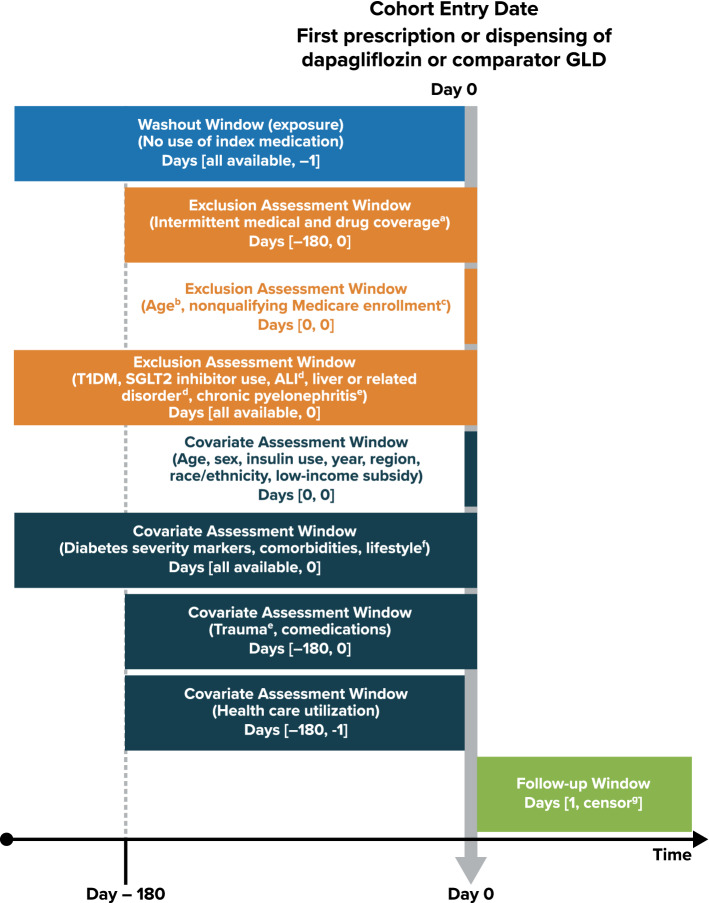
Fig. 1.
Study design, cohort eligibility, and inclusion criteria. CPRD Clinical Practice Research Datalink, GLD glucose-lowering drug, GOLD General Practitioner Online Database of the CPRD, hALI hospitalization for acute liver injury, HIRD HealthCore Integrated Research Database, SGLT2 sodium-glucose cotransporter 2, sUTI severe complications of urinary tract infection (pyelonephritis or urosepsis), T1DM type 1 diabetes mellitus. Schematic based on the framework of graphical representation for visualizing longitudinal study designs proposed by Schneeweiss et al [50]. The light blue box represents the assessment window for use of the index study medication, the orange boxes represent the assessment windows for the exclusion criteria, the dark blue boxes represent the assessment windows for covariate variables, and the green box represents the follow-up period. aCPRD, registered in an up-to-standard participating general medical practice. HIRD, complete pharmacy and medical coverage in a health insurance plan with no enrollment gaps greater than 30 days; Medicare, enrolled in fee-for-service insurance in Parts A (hospital insurance), B (medical insurance), and D (prescription drug coverage). bCPRD, ages < 18 years; HIRD, ages < 18 or > 64 years; Medicare, ages < 65 years. cMedicare, enrolled because of disability or end-stage renal disease; nonresident of a US state or the District of Columbia; enrolled in managed care coverage. dApplicable only to the cohorts assessing the hALI outcome. eApplicable only to the cohorts assessing the sUTI outcome. fBody mass index, smoking history, and alcohol use were available in CPRD GOLD data but were not available in HIRD or Medicare. gDiscontinuation of the study medication (30 days after the end of the days’ supply of the last consecutive prescription or dispensing), hALI or sUTI event, death, end of patient-specific data or eligibility in each data source or end of the study period, initiation of an SGLT2 inhibitor, diagnosis of T1DM