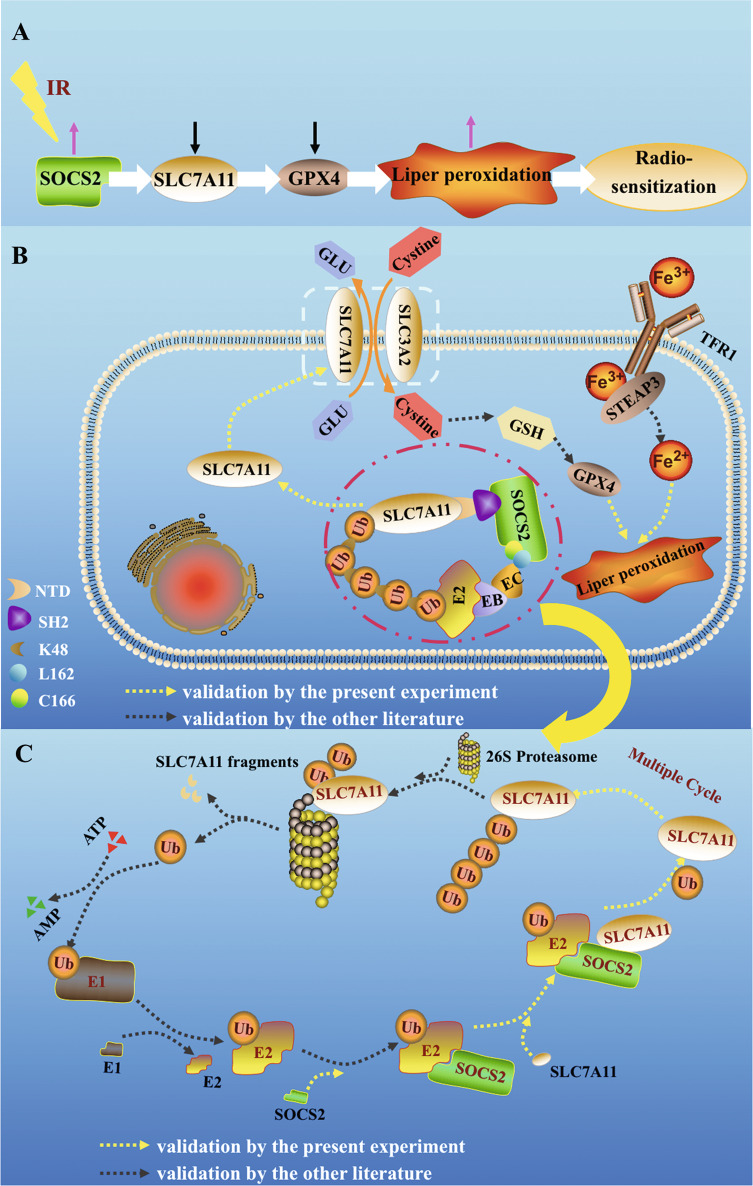
Fig. 8. Incremental SOCS2 promoted ubiquitination degradation of SLC7A11 and induced ferroptosis and radiosensitization of HCC.
A The pathway of SOCS2 sensitization. After IR, a rapid increase of SOCS2 led to a decrease of SLC7A11 and advanced ferroptosis and radiosensitization. B Diagram of the specific mechanism of SOCS2-induced ferroptosis. SOCS2-SH2 recognized SLC7A11-NTD, bound ubiquitin molecules which was linked to E2 ubiquitin-binding enzymes with the joint involvement of elongin B (EB) and elongin C (EC), and facilitated the transfer of ubiquitin molecules to the substrate protein SLC7A11 to promote SLC7A11 degradation. This degradation subsequently caused a reduction in cystine intake as well as a reduction in GSH and GPX4 and ultimately advanced ferroptosis. C The processes of SLC7A11 protein ubiquitination. In an ATP-dependent reaction, Ub was attached to the ubiquitin-activating enzyme E1 and activated, then delivered to the ubiquitin-binding enzyme E2. Next, the E3 ligase SOCS2 acted as a bridge, identifying E2-Ub and the substrate SLC7A11 to facilitate the association of Ub with SLC7A11. After several repetitions of the above process, a K48-ubiquitin chain was formed and attached to SLC7A11. Finally, the K48-ubiquitin chain was identified by the 26 S proteasome, leading to the degradation of SLC7A11 to fragments. Yellow arrows indicate the experimental results argued in this paper while black arrows represent results reported in other literature.