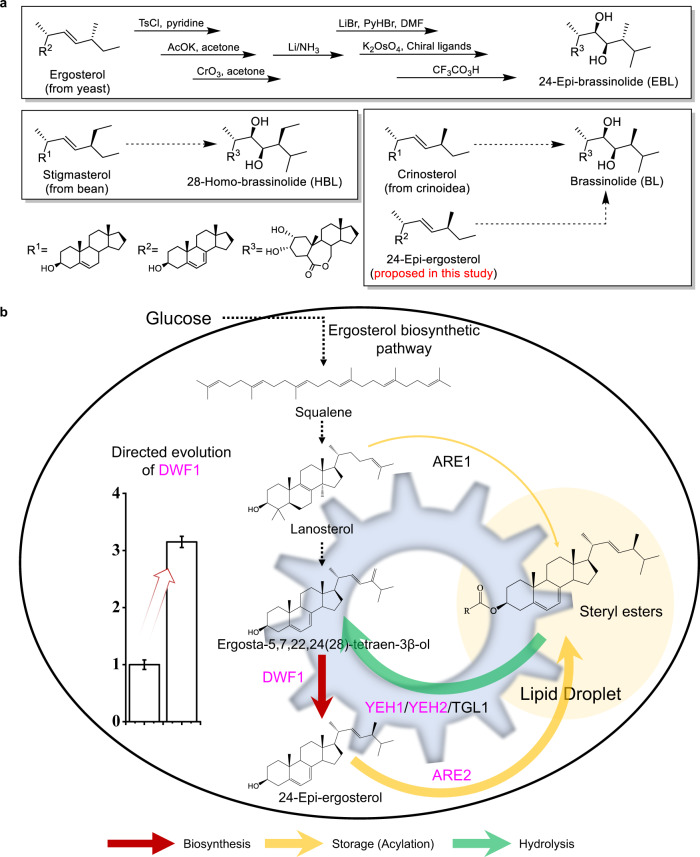
Fig. 1. Construction of engineered yeast strains for de novo biosynthesis of 24-epi-ergosterol, serving as a synthetic precursor for BL.
a Semi-synthesis of 24-epi-brassinolide (EBL), 28-homo-brassinolide (HBL), and brassinolide (BL) from ergosterol, stigmasterol, and crinosterol, respectively. In comparison with crinosterol, 24-epi-ergosterol, the diastereoisomer to ergosterol, could be produced on a large scale using yeast strains constructed in the present study. b Manipulation of sterol homeostasis for the production of 24-epi-ergosterol in yeast. The introduction of a Δ24(28)-sterol reductase (DWF1) from plants enabled de novo biosynthesis of 24-epi-ergosterol. Afterward, the catalytic activity of DWF1 and, accordingly, the production of 24-epi-ergosterol was enhanced by directed evolution. The sterol fluxes towards 24-epi-ergosterol were further strengthened by the engineering of sterol homeostasis, maintaining a balance between sterol acylation (for storage in LDs) and steryl ester hydrolysis (for releasing free sterols in cellular membranes) via overexpression of YEH1, YEH2, and ARE2 with intact ARE1. DWF1 Δ24(28)-sterol reductase, ARE1 sterol O-acyltransferase 1, ARE2 sterol O-acyltransferase 2, YEH1 yeast steryl ester hydrolase 1, YEH2 yeast steryl ester hydrolase 2, TGL1 triglyceride lipase.