Figure 1.
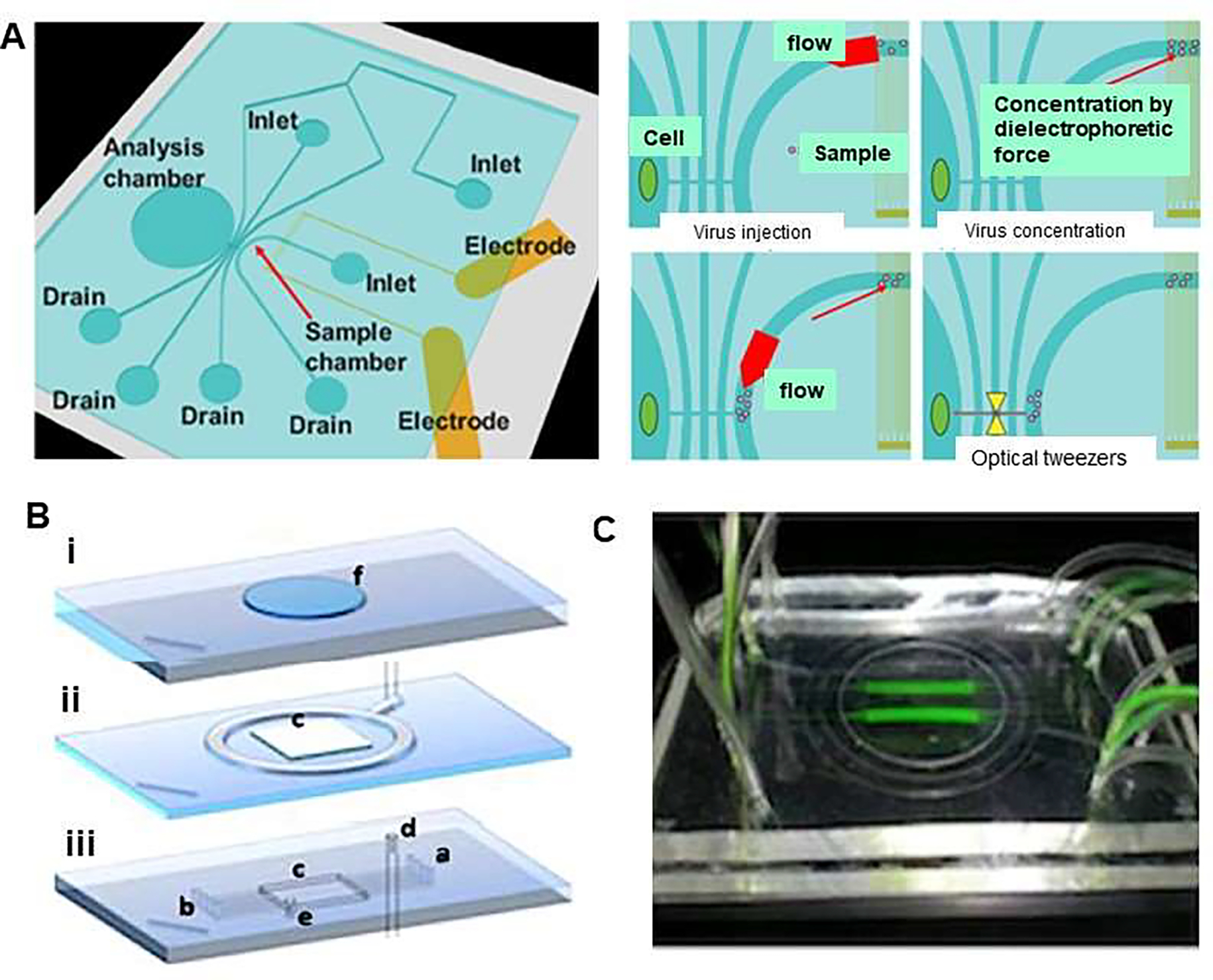
Viral concentration and optical tweezers in a chip. (A) Schematic illustration represents single cell infection to a specific cell applying dielectrophoretic (DEP) virus concentration and optical tweezers in a chip. Virus solution was injected to the sample chamber combined with the injection of buffer as sheath flow and were concentrated by DEP force. The conductivity of the solution was adjusted to 10 mS/m to prevent heat damage for DEP concentration and virus were gathered by negative DEP force. In addition, DEP force avoided the adhesion of virus to the chip, then selected viruses were trapped and transported to the analysis chamber by optical tweezers. Reproduced from [26], with permission from the Institute of Electrical and Electronics Engineers (IEEE). (B) Design of microfluidic platform which comprises (i) a supporting glass slide that has a carved polydimethylsiloxane (PDMS) slab to accommodate cell culture coverslip, (ii) a membrane-based vacuum system to achieve reversible sealing of component (i), and (iii) 0.2×0.1mm (w×h) microfluidic channels, which provide cultured cells with fluids (a: inlet, outlet: b). The platform forms a 16×16×0.5mm culture chamber (c). The embedding top layer is connected to the vacuum system (d) and to a pressure-monitoring auxiliary service (e). (C) An image of the platform, which is transparent, showing flowing tracer (fluorescein) in two of the eight channels allowing for several levels of virus (MOIs) at a single time point. Reproduced from [27], with permission from the American Institute of Physics (B&C).
