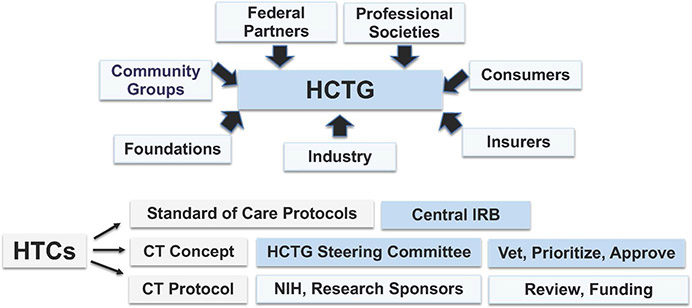
FIGURE 4.
Haemophilia Clinical Trials Group Infrastructure. The haemophilia clinical trials group (HCTG) infrastructure was modelled after the Children's Oncology Group (COG), in which clinical trials are conducted within the organizational structure of the haemophilia treatment centre (HTC), supported by federal partners, foundations, industry, insurers, consumers and professional societies. Within HCTG, HTC MDs develop standard of care protocols and intervention trial concepts which are approved by a single IRB. Trial concepts are vetted and prioritized by the HCTG Steering Committee, and approved concepts are developed into protocols, which are submitted to NIH and other research sponsors for review and funding recommendations