Table 9.
Comparison of sensitivity and greenness report between the suggested and reported HPLC methods.
| Method | Linearity range μg/mL |
Studied drugs | Mobile phase | Run time (min.) | Flow rate (mL/min.) | Waste (g/run) Run time × flow rate10 |
NEMI | Eco-scale score | Complex GAPI | AGREE |
|---|---|---|---|---|---|---|---|---|---|---|
|
Reported HPLC method |
5 to 30 diethyl-carbamazine 0.1 to 1 LVC | diethylcarbamazine and LVC | potassium dihydrogen orthophosphate buffer (pH: 3.2): acetonitrile (50:50 v/v) | 6 | 1 | 6 |

|
66 |
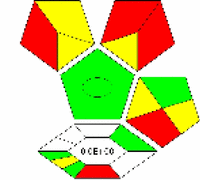
|
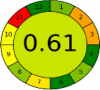
|
|
Reported HPLC method |
5–35 for all the drugs | Salbutamol sulfate, Guaifenesin, and AMB | potassium dihydrogen ortho-phosphate buffer and methanol (58:42 v/v), pH 4.5 | 7 | 1 | 7 |

|
70 |
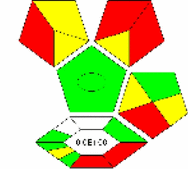
|
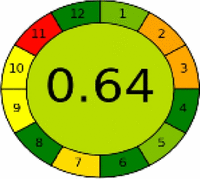
|
|
Reported HPLC method |
2.5–10 PHN, 250–750 PAR, 15–45 caffeine and 1.25–3.54 LVC | PHN, PAR, LVC and caffeine | Three steps gradient elution, 10 mM phosphate buffer (pH 3.3) and methanol 2–80% | 18 | 1 | 18 |

|
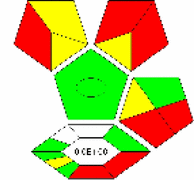
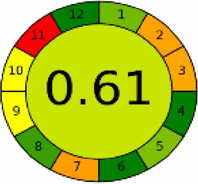
67 |
|
|
|
Reported HPLC method |
50–150% for all the drugs | Ascorbic acid, PHN, PAR and LVC | A gradient elution using phosphate buffer pH 4.0: Acetonitrile | 23 | 1.5 | 34.5 |

|
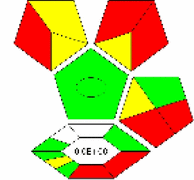
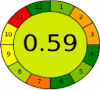
64 |
|
|
|
Reported HPLC method |
Not available | chlorpheniramine maleate, PAR and PHN | acetonitrile: a buffer solution of 0.01 M dipotassium phosphate and monopotassium phosphate (50:50), pH 7.8 | 14 | 1 | 14 |

|
65 |
|
|
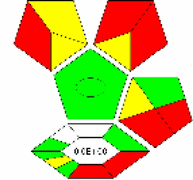
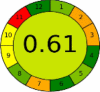
| Reported HPLC method22 | 250–750 PAR, 1.25–3.75 LVC, 2.5–7.5 PHN and 30–90 AMB | PAR, LVC, PHN and AMB | Acetonitrile and Phosphate buffer, pH 3.5, three steps gradient elution | 17.9 | 1.0 | 17.9 |

|
76 |
|
|
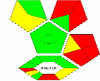
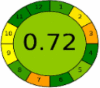
| Reported HPLC method23 | 162.5–975 PAR, 2.5–15 LVC, PHN and 15–90 AMB | PAR, LVC, PHN and AMB | Methanol: Sodium Phosphate dibasic anhydrous Buffer (65:35 V/V), pH of buffer 7.0 ± 0.02 | 12.3 | 1.0 | 12.3 |

|
79 |
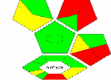
|
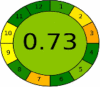
|
| Proposed HPLC method |
50 -500 PAR 0.5–20 LVC, PHN and 0.5–100 AMB |
PAR, LVC, PHN and AMB | Ethanol: acetonitrile: 2.5 mM HSA (20:5:75), pH 6.5 | 9 | 1.0 | 9 |

|
89 |
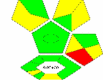
|
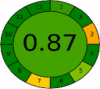
|
