Scheme 5. Synthesis of Carboxamide N-Alkylated Derivatives.
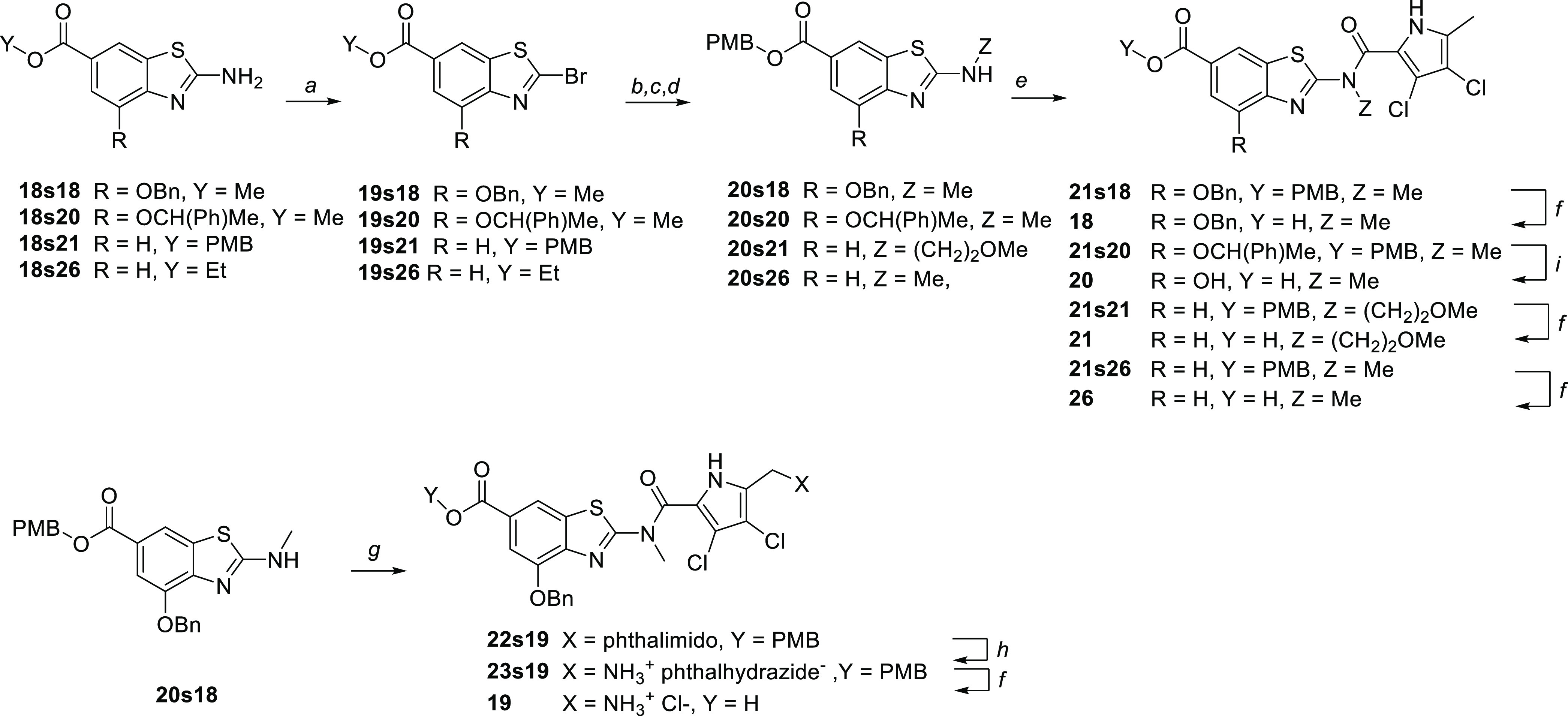
Reagents and conditions: (a) CuBr2, t-BuONO, CH3CN, 22 °C, 15 h; (b) Z-NH2, THF, 22 °C, 15 h; (c) 2 M NaOH, 1,4-dioxane, 50 °C, 24 h (for 20s18, 20s20, 20s26); (d) p-methoxybenzyl chloride, K2CO3, DMF, rt, 15 h (for 20s18, 20s20, 20s26); (e) 3,4-dichloro-5-methyl-1H-pyrrole-2-carbonyl chloride, toluene, 130 °C, 15 h; (f) 1 M HCl in AcOH, 22 °C, 18 h; (g) 3,4-dichloro-5-phthalimidomethyl-1H-pyrrole-2-carbonyl chloride,36 toluene, 130 °C, 15 h; (h) (1) hydrazine hydrate, EtOH, 50 °C, 40 min; (2) HCl, MeOH, 22 °C, 15 min; (3) EtOH, reflux, 18 h; and (i) SnCl4, DCM, 22 °C, 2 h.
