Scheme 8. Synthesis of Branched 4-Alkoxy Analogs Containing a Polar Group.
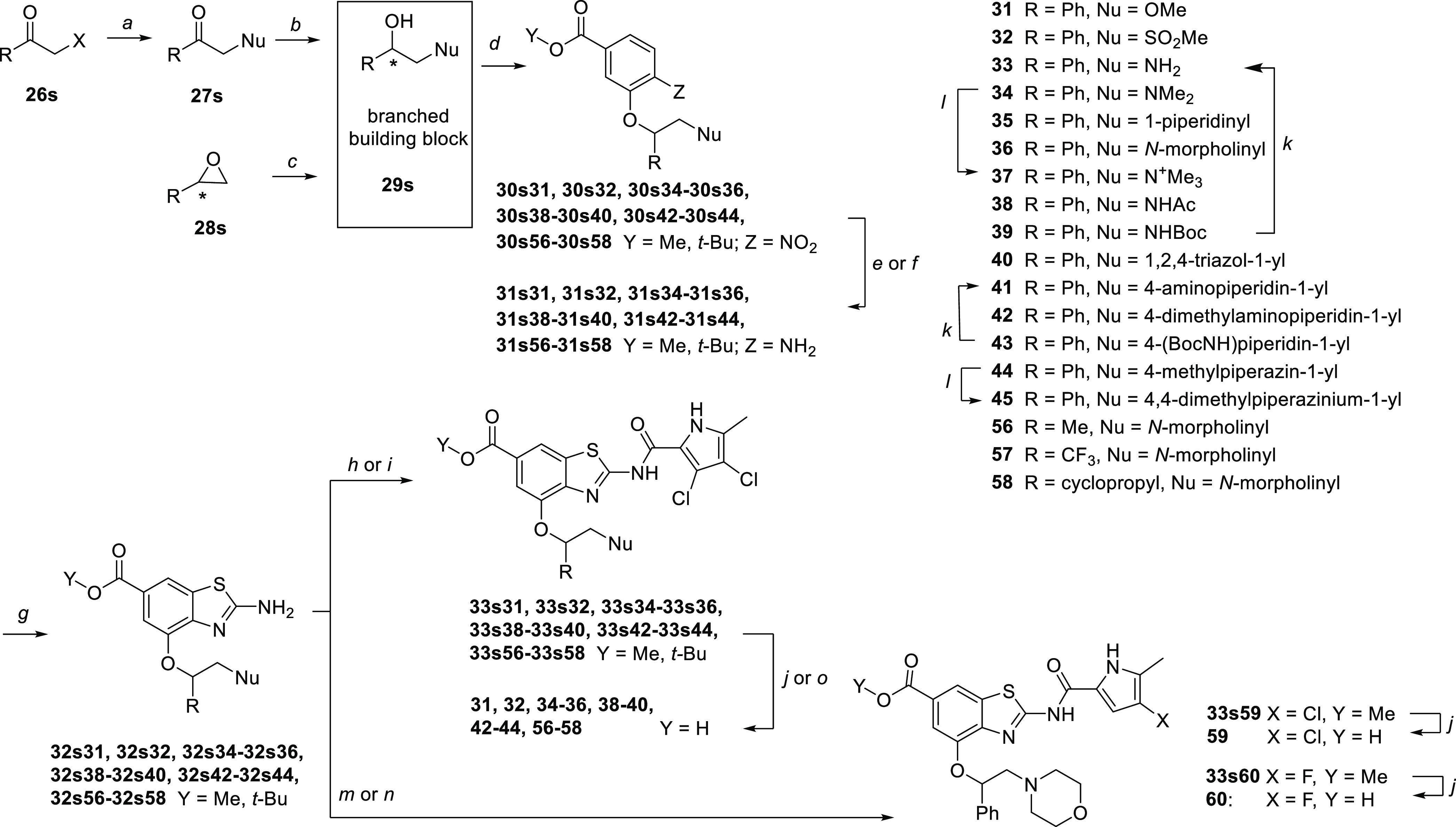
Reagents and conditions: (a) nucleophile, K2CO3, KI, MeCN, 22 °C, 24 h; (b) NaBH4, MeOH, 0–22 °C, 2 h; (c) nucleophile, DMF or neat, heating; (d) 2s, PPh3, DIAD, THF, 22 °C, 18 h; (e) Fe, AcOH, 22 °C, 2 h; (f) H2, Pd/C, MeOH, 22 °C, 5 h; (g) KSCN, Br2, AcOH, 22 °C, 15 h; (h) 3,4-dichloro-5-methyl-1H-pyrrole-2-carbonyl chloride, toluene, 130 °C, 15 h; (i) 2-trichloroacetyl-3,4-dichloro-5-methyl-1H-pyrrole, Na2CO3, DMF, 65 °C, 24 h; (j) 2 M NaOH, MeOH, 40 °C, 48 h; (k) 1 M HCl in 1,4-dioxane, 22 °C, 24 h; (l) MeI, THF, 60 °C, 24 h; (m) 2-trichloroacetyl-4-chloro-5-methyl-1H-pyrrole, Na2CO3, DMF, 65 °C, 18 h; (n) 4-fluoro-5-methyl-1H-pyrrole-2-carbonyl chloride, toluene, 130 °C, 18 h; and (o) CF3COOH, dichloromethane, 22 °C, 18 h.
