Extended Data Fig. 8 |. Comparison of the Und-PP-bound and the apo-CmWaaL structures.
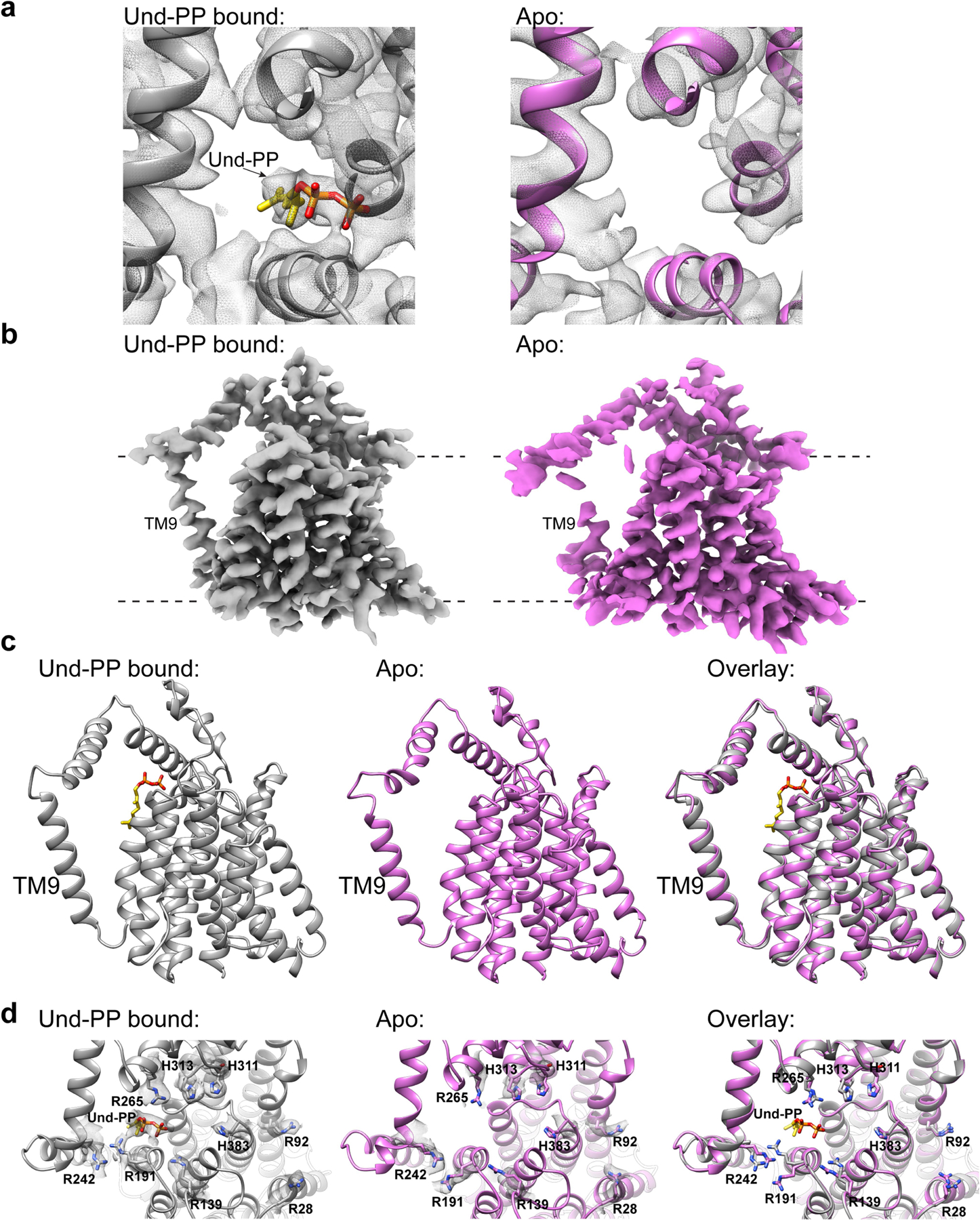
a, Top Views of the Und-PP bound (grey) and the apo (pink) CmWaaL structures, showing the density (mesh) of the Und-PP (yellow) in the ligand-bound structure in comparison to the apo structure. b, Cryo-EM density maps for the Und-PP-bound (grey) and the apo (pink) CmWaaL. Density maps were prepared in chimeraX55, by deleting any density within a 4 Å radius of the Fab in the final model. c, Side views of the Und-PP-bound (grey) and the apo (pink) CmWaaL shown as ribbon. The Und-PP (yellow) is shown as sticks in the bound structure. d, Top views of the Und-PP-bound (grey) and the apo (pink) CmWaaL showing the key residues that we hypothesize play a role in either binding/shuttling or ligation of the substrates. The density for the selected residues is shown as grey mesh. On the right an overlay of bound and apo states are shown with highlighted residues shown in stick representation.
