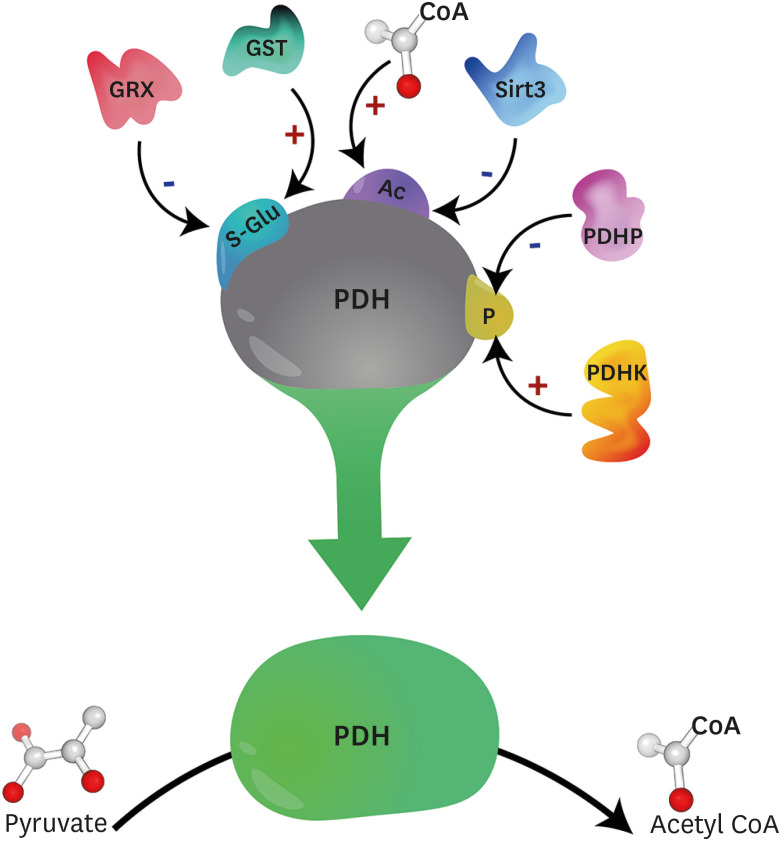
Fig. 2. Post-translational modifications impacting PDH activity. PDH activity is subject to numerous post-translational modifications, including phosphorylation, which is the best characterized. PDHK mediated phosphorylation inhibits PDH activity, whereas PDHP mediated dephosphorylation stimulates PDH activity. PDH may also be inactivated by acetylation, with recent studies suggesting that increases in mitochondrial acetyl CoA increase mitochondrial protein acetylation nonenzymatically, whereas Sirt3 has been demonstrated to increase PDH activity via deacetylation. Finally, PDH is also subject to S-glutathionylation mediated inactivation, though the role of GST and GRX in catalyzing and removing S-glutathionylation modifications of PDH, respectively, remains to be determined.
PDH, pyruvate dehydrogenase; PDHK, pyruvate dehydrogenase kinase; PDHP, pyruvate dehydrogenase phosphatase; CoA, coenzyme A; Sirt, sirtuin; GST, glutathione-S-transferases; GRX, glutaredoxins.