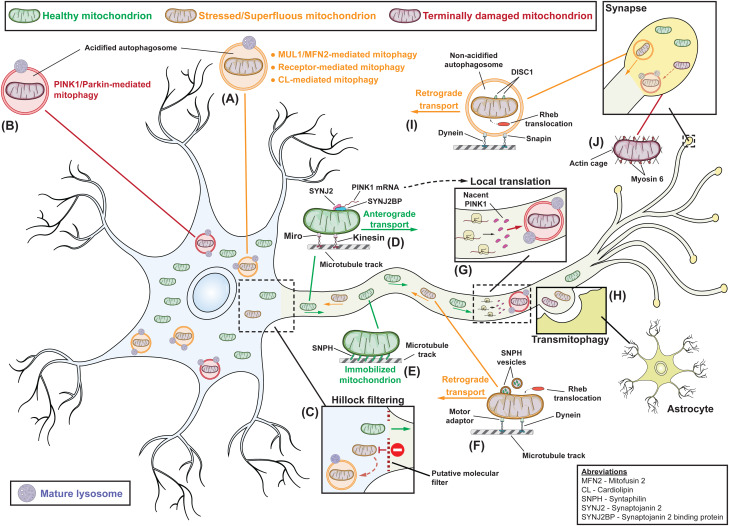
Fig. 2.
Putative integrated model for the compartmentalisation of mitophagy in neurons. The turnover of stressed or superfluous mitochondria in the main cell body of neurons is predominantly mediated by PINK1-independent mechanisms, which involve the action of mitophagy receptors, cardiolipin and the ubiquitination of MFN2 by MUL1 (A), while terminally damaged organelles are cleared through the PINK1/Parkin pathway (B). Quality control mechanisms at the axonal hillock (C) filter organelles and only allow healthy mitochondria to travel anterograde along microtubule tracks (D), whereas damaged ones are targeted for degradation. Along the axon, the anchoring protein syntaphilin (SNPH) antagonises motor proteins to promote the immobilisation of functional organelles at areas of high mitochondrial demand (E). Rheb small GTPase translocates on stressed axonal mitochondria, which release the anchoring protein SNPH and are remobilised in order to travel retrograde and be degraded in the soma (F). Terminally damaged axonal organelles are degraded locally through the PINK1/Parkin pathway, which is supported by local PINK1 mRNA translation (G). Damaged mitochondria that fail to travel retrograde or be locally degraded can be externalised and cleared by neighbouring astrocytes though the process of transmitophagy (H). At synapses, Rheb translocation induces DISC1-mediated formation of autophagosomes around stressed mitochondria, that remain non-acidified and interact with the dynein adaptor protein Snapin to travel back to the soma (I). Terminally damaged synaptic mitochondria acquire myosin 6, which promotes the assembly of stable actin cages and immobilises organelles to enable their local Parkin-mediated turnover (J). Somatodendritic regions are marked with light blue, axonal regions with light green and synapses with yellow background.