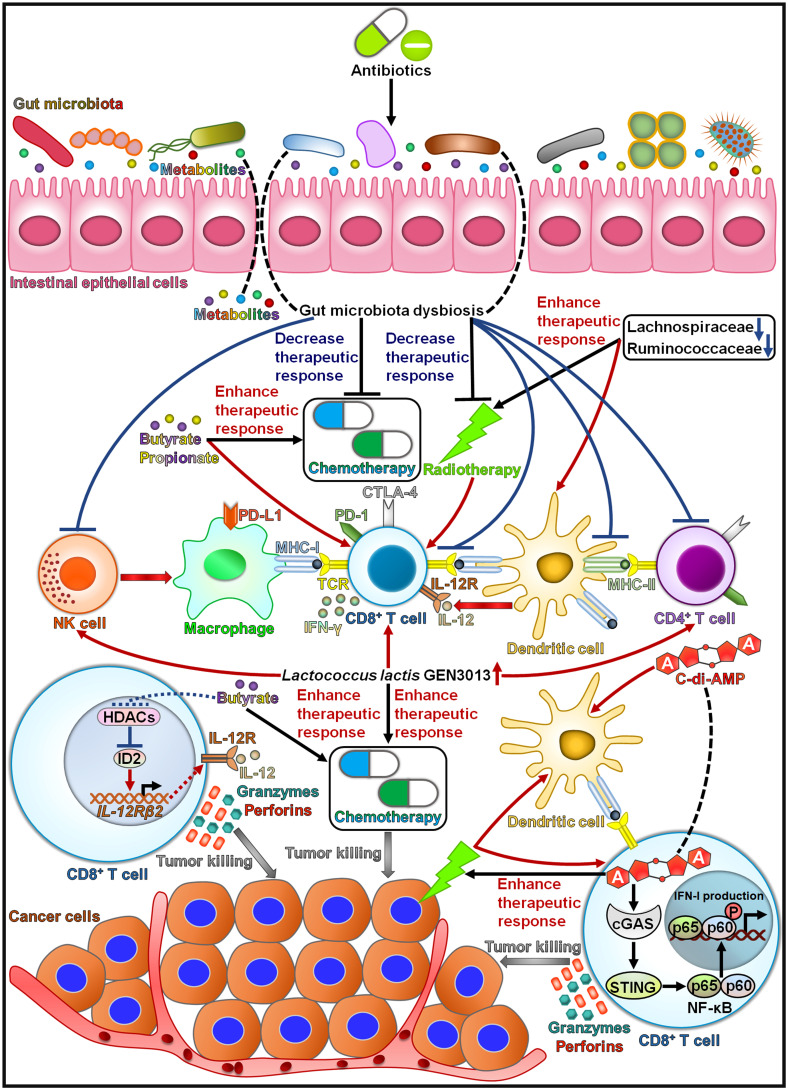
Figure 3.
The impact of the gut microbiota on the responses to cancer chemotherapy and radiotherapy. Antibiotic-induced intestinal dysbiosis contributes to decreased therapeutic response to anticancer agents through inhibition of immune cells including NK cells, dendritic cells and CD4+ T cells. Depletion of Lachnospiraceae and Ruminococcaceae potentiates the anticancer efficacy of radiotherapy by enhancing antigen presentation and CD8+ T cell activity. Gut microbial metabolites butyrate and propionate enhance the anticancer activity of oxaliplatin by inducing CD8+ T cell responses. Butyrate can downregulate HDACs to increase ID2 expression, leading to the activation of IL-12 signaling pathway. These events cause the reinforcement of CD8+ T cell function and improvement of chemotherapeutic efficacy. Lactococcus lactis GEN3013 increases the effectiveness of conventional chemotherapy by promoting the accumulation of NK cells, CD4+ T cells and CD8+ T cells. Gut microbiota dysbiosis suppresses the activation of T cells induced by radiotherapy, hence restricting the responsiveness to radiotherapy. C-di-AMP synergizes with radiotherapy to facilitate the maturation and presentation functions of DCs, enhancing IFN-β production and CD8+ T cell activation via the cGAS/STING signaling cascade. NK cell, natural killer cell; PD-L1, programmed death-ligand 1; MHC-I, major histocompatibility complex class I; IFN-γ, interferon-γ; TCR, T cell receptor; PD-1, programmed death-1; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; IL-12, interleukin-12; IL-12R, interleukin-12 receptor; MHC-II, major histocompatibility complex class II; HDACs, histone deacetylases; ID2, Inhibitor of DNA binding 2; IL-12Rβ2, interleukin-12 receptor β2; c-di-AMP, cyclic di-adenosine monophosphate; cGAS, cyclic GMP-AMP synthase; STING, stimulator of interferon genes; NF-κB, nuclear factor-κB.