Abstract
Background:
Non-Thyroidal Illness Syndrome (NTIS) caused by infection or fasting is hallmarked by reduced circulating thyroid hormone (TH) levels. To better understand the role of local TH-action in the development of NTIS, we assessed tissue-specific changes of TH signaling in Thyroid Hormone Action Indicator (THAI) mice.
Methods:
NTIS was induced in young adult THAI mice by bacterial lipopolysaccharide (LPS)-administration or by 24 or 48 hours' fasting. Tissue-specific TH-action was assessed by the detection of changes of the Luciferase reporter of THAI mice with quantitative polymerase chain reaction along with tissue-specific examination of regulators of TH metabolism and signaling. Age dependence of revealed alterations of hypothalamic TH-action was also studied in 1-year-old male THAI mice.
Results:
LPS-treatment increased TH-action in the hypothalamic arcuate nucleus-median eminence (ARC-ME) region preceded by an increase of type 2 deiodinase (D2) expression in the same region and followed by the suppression of proTrh expression in the hypothalamic paraventricular nucleus (PVN). In contrast, LPS decreased both TH-action and D2 activity in the pituitary at both ages. Tshβ expression and serum free thyroxine (fT4) and free triiodothyronine (fT3) levels decreased in LPS-treated young adults. Tshβ expression and serum fT4 levels were not significantly affected by LPS treatment in aged animals. In contrast to LPS treatment, TH-action remained unchanged in the ARC-ME of 24 and 48 hours fasted animals accompanied with a modest decrease of proTrh expression in the PVN in the 24-hour group. Tshβ expression and fT3 level were decreased in both fasted groups, but the fT4 decreased only in the 48 hours fasted animals.
Conclusions:
Although the hypothalamo-pituitary-thyroid (HPT) axis is inhibited both in LPS and fasting-induced NTIS, LPS achieves this by centrally inducing local hyperthyroidism in the ARC-ME region, while fasting acts without affecting hypothalamic TH signaling. Lack of downregulation of Tshβ and fT4 in LPS-treated aged THAI mice suggests age-dependent alterations in the responsiveness of the HPT axis. The LPS-induced tissue-specific hypo-, eu-, and hyperthyroidism in different tissues of the same animal indicate that under certain conditions TH levels alone could be a poor marker of tissue TH signaling. In conclusion, decreased circulating TH levels in these two forms of NTIS are associated with different patterns of hypothalamic TH signaling.
Keywords: aging, fasting, hypothalamo-pituitary-thyroid axis, Non-Thyroidal Illness Syndrome, Thyroid Hormone Action Indicator Mouse, tissue-specific thyroid hormone action
Introduction
The Non-Thyroidal Illness Syndrome (NTIS) is caused by fasting or serious disease conditions such as infection and hallmarked by central inhibition of the hypothalamo-pituitary-thyroid (HPT) axis.1–3 Similar to humans, fasting and bacterial lipopolysaccharide (LPS) administration, a model of infection, also causes NTIS in rodents.4–7
Despite decreased circulating thyroid hormone (TH) levels, we have suggested that LPS-induced increase of the activity of the TH activating type 2 deiodinase (D2) enzyme in tanycytes of the mediobasal hypothalamus (MBH) may cause local hyperthyroidism in the MBH and therefore may be a key mediator of the effect of LPS on HPT axis suppression.4 This hypothesis was, however, debated by Mebis et al based on the absence of increased triiodothyronine (T3) content in the whole hypothalamus of a rabbit NTIS model.8 As the available methods were not sufficiently sensitive to determine the T3 content of the very small MBH, our hypothesis could not be directly proven.
Diano et al observed a twofold increase of D2 activity also in the MBH of fasted rats and suggested that increased TH activation in the MBH could contribute to fasting-induced inhibition of the HPT axis.9
To overcome the challenge of the direct assessment of tissue-specific TH-action, we generated the Thyroid Hormone Action Indicator (THAI) mouse model that allows tissue-specific detection of TH-action and gauges TH signaling within a physiological context.10 To better understand the development of NTIS, we studied in THAI mice whether local TH-action is changed in the MBH and could contribute to the development of LPS- and fasting-induced NTIS along with measuring tissue-specific TH-action and parameters of the HPT axis. The impact of aging was also studied on the revealed alterations of hypothalamic TH signaling.
Materials and Methods
Methods are detailed in Supplemental Data.
Animals
We used 3-month-old (young adult) and 1-year-old (aged) male THAI line #23 FVB/Ant mice that allows tissue-specific assessment of TH-action.10 In this transgenic mouse model, the expression of the luciferase reporter is driven by a minimal viral promoter that was made TH sensitive by the insertion of three copies of the TH response element of the human dio1 gene.10 Animals were housed under standard environmental conditions (light between 06:00 and 18:00 hours, temperature 22 ± 1°C, mouse chow, and water ad libitum). All experimental protocols were reviewed and approved by the Animal Welfare Committee at the Institute of Experimental Medicine, Hungary (PEI/001/1550-10/2014 - PE/EA/1490-7/2017).
LPS treatment, fasting, and microdissection of brain areas
Mice were treated i.p. with LPS (150 μg/animal; Escherichia coli 0127:B8; Sigma) or saline as control and decapitated 6, 8, and 10 hours after injection (animal number is given in the legends). Treatment time was optimized based on preliminary experiments between 4 and 24 hours; 6–10 hours was found to be appropriate to observe the well-known hallmarks of acute NTIS. Animals were fasted for 24 or 48 hours followed by tissue collection and microdissection.11
Taqman real-time PCR
Reverse transcription-quantitative polymerase chain reaction was performed on Viia 7 Real-time PCR instrument (Applied Biosystems). Accession numbers of used Taqman probes are listed in Supplementary Table S1. The sequence of the dCpG Luciferase probe has been previously published.10
ProTrh messenger RNA in situ hybridization
Hybridization was performed using a 741 base (corresponding to the 106–846 nucleotides of the mouse proTrh messenger RNA [mRNA]; BC053493) single-stranded [35S]UTP labeled cRNA probe for mouse proTrh as previously described.12
Deiodination assay
D2 activity of whole pituitary samples was assayed in the presence of 125I-thyroxine (T4) and released iodine was counted, as previously described.13
Determination of circulating hormone levels
Serum free T4 (fT4), free T3 (fT3), and serum thyrotropin (TSH) were determined as provided in Supplementary Data.
Simple Western blot (WES)
Thyrotropin-releasing hormone-degrading ectoenzyme (TRH-DE) content of microdissected arcuate nucleus-median eminence (ARC-ME) samples was measured with WES Simple Western capillary electrophoresis system.
Data analysis
Student's two-sample two-sided t-test was used to analyze two groups; one-way analysis of variance followed by Tukey and Dunnett post hoc test was used to compare more than two groups.
Results
TH signaling undergoes marked changes in NTIS in regulatory regions of the HPT axis
In the PVN of young adult THAI mice, the expression of proTrh mRNA was significantly decreased between 6 and 8 hours (Fig. 1A). The LPS-treatment also reduced the Tshβ mRNA level in the pituitary (Fig. 1B), and serum TSH level was also decreased at 10 hours (Supplementary Fig. S1). In the serum, circulating fT4 and fT3 levels were markedly decreased (Fig. 1C, D) as expected.4,5
FIG. 1.
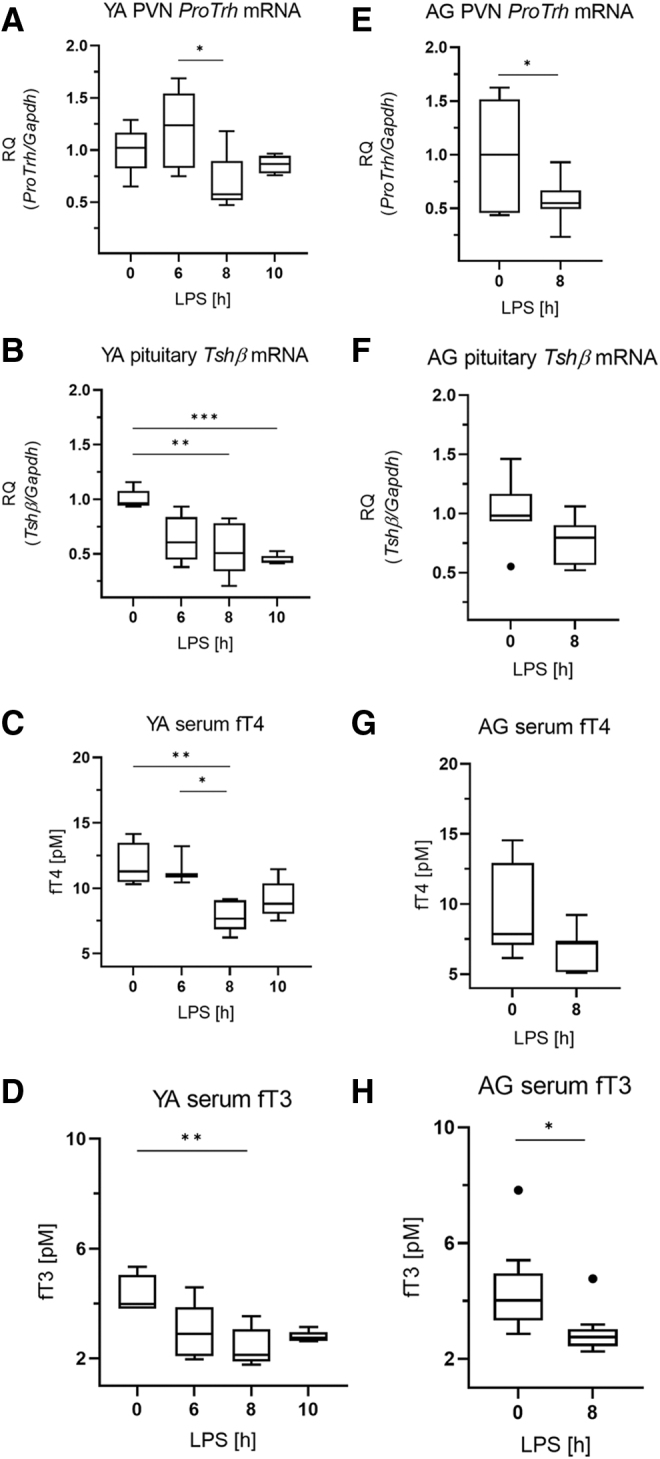
Age-dependent responsiveness of the HPT axis in LPS-induced NTIS. Male THAI mice were treated with 150 μg/animal LPS i.p. (A–D) YA (2–3 months old), (A) PVN proTrh mRNA levels levels, (B) pituitary Tshβ mRNA levels, (C) serum fT4 levels, (D) serum fT3 levels, (E–H) AG (1 year old), (E) PVN proTrh mRNA levels, (F) pituitary Tshβ mRNA levels, (G) serum fT4 levels, (H) serum fT3 levels n(YA) = 5/group. n(AG) = 6–9/group; figure shows Tukey Box Plots, α = 0.05; *p < 0.05, **p < 0.01, ***p < 0.001. AG, aged adults; fT3, free triiodothyronine; fT4, free thyroxine; HPT, hypothalamo-pituitary-thyroid; LPS, lipopolysaccharide; mRNA, messenger RNA; NTIS, Non-Thyroidal Illness Syndrome; PVN, paraventricular nucleus; RQ, relative quantity; THAI, Thyroid Hormone Action Indicator; YA, young adults.
Earlier, we showed that LPS-treatment results in a marked increase of tanycytic D2 activity that is independent from decreased peripheral TH levels.4,14 Based on this, we hypothesized that the increased TH activating capacity of tanycytes induces local hyperthyroidism in the MBH despite the lower serum fT4 levels, and it inhibits the hypophysiotropic TRH neurons by enhancing negative TH feedback.4,14 To determine how LPS-treatment impacts local TH economy in the MBH, we tested the effect of LPS-treatment on TH-action in the ARC-ME region using the THAI mouse model.
The LPS-treatment resulted in a time-dependent elevation of Luciferase expression (marker of TH-action) in the ARC-ME region that became statistically significant 8 hours after LPS injection (Fig. 2A). The observed increase of local TH signaling was preceded by a marked increase of D2 expression in the ARC-ME region (6 hours after LPS-treatment) (Fig. 2B).
FIG. 2.
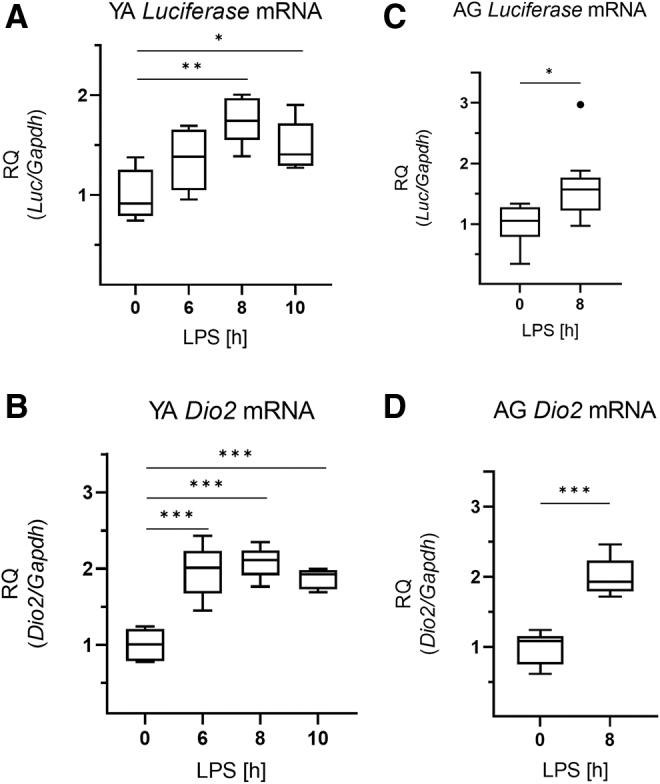
Thyroid hormone action in the hypothalamus in LPS-induced NTIS. Male THAI mice were treated with 150 μg/animal LPS i.p.; Luciferase expression represents local TH-action. (A, B) YA (2–3 months old), (A) ARC-ME Luciferase mRNA levels, (B) ARC-ME Dio2 mRNA levels, (C, D) AG (1 year old), (C) ARC-ME Luciferase mRNA levels, (D) ARC-ME Dio2 mRNA levels. n(YA) = 5/group; n(AG) = 8–9/group; figure shows Tukey Box Plots, α = 0.05; *p < 0.05, **p < 0.01, ***p < 0.001. ARC-ME, arcuate nucleus-median eminence; TH, thyroid hormone.
In contrast to the hypothalamus, LPS-treatment decreased Luciferase expression in the pituitary, indicating a reduction of TH signaling in this tissue (Fig. 3A). This was accompanied with decreased D2-mediated TH activation and decreased D3-mediated TH inactivation (Fig. 3B, C, respectively). Expression of TH transporters Oatp1c1 (Slco1c) and Mct8 (Slc16a2) remained unchanged in the pituitary (Fig. 3D, E).
FIG. 3.
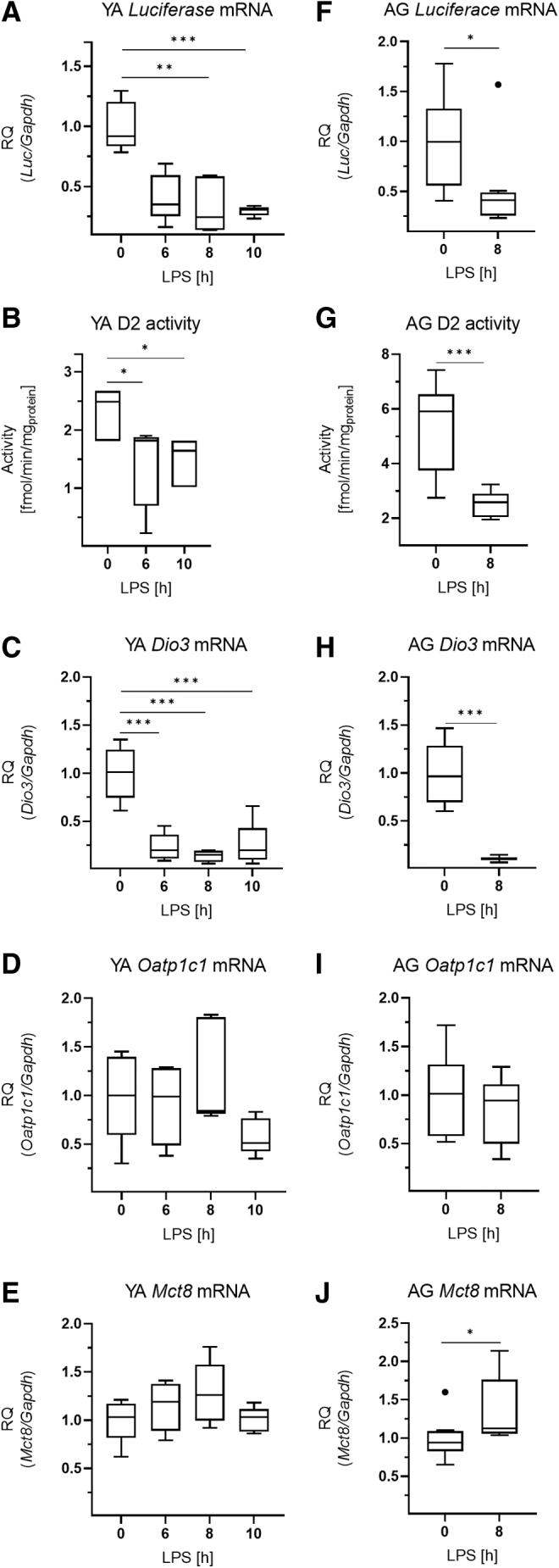
Thyroid hormone action in the pituitary in LPS-induced NTIS. Male THAI mice were treated with 150 μg/animal LPS i.p.; Luciferase expression represents local TH-action. (A–E) YA (2–3 months old), (A) Luciferase mRNA levels, (B) D2 activity, (C) Dio3 mRNA levels, (D) Oatp1c1 mRNA levels, (E) Mct8 mRNA levels (F–J) AG (1 year old), (F) Luciferase mRNA levels, (G) D2 activity, (H) Dio3 mRNA levels, (I) Oatp1c1 mRNA levels, (J) Mct8 mRNA levels. n(YA) = 5/group; n(AG) = 7–9/group; figure shows Tukey Box Plots, α = 0.05; *p < 0.05, **p < 0.01, ***p < 0.001. D2, type 2 deiodinase.
Responsiveness of the HPT axis is age-dependent in NTIS
Since TH economy is subjected to age-mediated changes,15 we also performed studies in aged adult (1 year old) THAI animals to study whether the revealed changes of TH signaling in NTIS are age-dependent. In 1-year-old mice, a similar decrease of proTrh mRNA level was observed in the PVN after LPS-treatment compared with young adults (Fig. 1E), indicating that the hypothalamic response of the HPT axis was age-independent.
However, in contrast to young adults, neither the expression of Tshβ in the pituitary nor the circulating fT4 levels was significantly decreased in aged animals (Fig. 1F, G). Serum TSH of aged animals was significantly lower in the controls compared with young adults (1.02 vs. 0.37, p = 0.001), and TSH levels of the LPS-treated aged group were below the limit of detection. Therefore, the magnitude of the impact of LPS on serum TSH could not be compared in an age-dependent manner.
fT3 levels were significantly decreased by LPS-treatment in both age groups (Fig. 1D, H). Similar to young adults, the LPS-induced increases of Luciferase and Dio2 expressions were also observed in the ARC-ME region of aged animals (Fig. 2C, D). In the pituitary of aged animals, the findings on Luciferase, D2 activity, Dio3, and Oatp1c1 (Fig. 3F–I) were similar to young adults, except that Mct8 expression was slightly increased in the aged group (Fig. 3J).
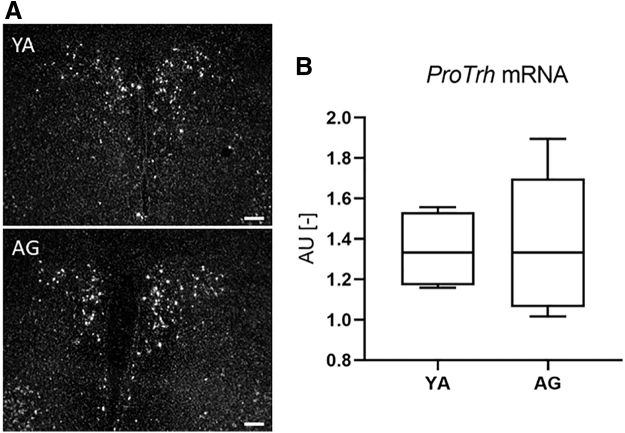
To understand the mechanisms underlying the observed age-related difference in Tshβ regulation, we studied factors with potential to alter central components of the HPT axis. ProTrh mRNA expression in the PVN of young adults and aged animals was compared by in situ hybridization, and no significant difference could be found (p = 0.099) (Fig. 4A, B). The major regulator of TRH peptide degradation, the Trh-de was also studied. The Trh-de mRNA was significantly decreased 8 hours after the LPS injection in the ARC-ME samples of both age groups, and this drop in Trh-de expression was significantly higher in the aged animals (0.48 vs. 0.60, p = 0.021) (Fig. 5A, D).
FIG. 4.
proTrh expression in the PVN of YA and aged THAI mice. (A) Representative dark-field micrographs of proTrh in situ hybridization at the mid-level PVN of YA (3 months old, upper panel) and AG (1 year old, lower panel) THAI mice. (B) Quantification of the proTrh mRNA hybridization signal. n(YA) = 4/group; n(AG) = 5/group; figure shows Tukey Box Plots, α = 0.05; Scale bar: 100 μm.
FIG. 5.
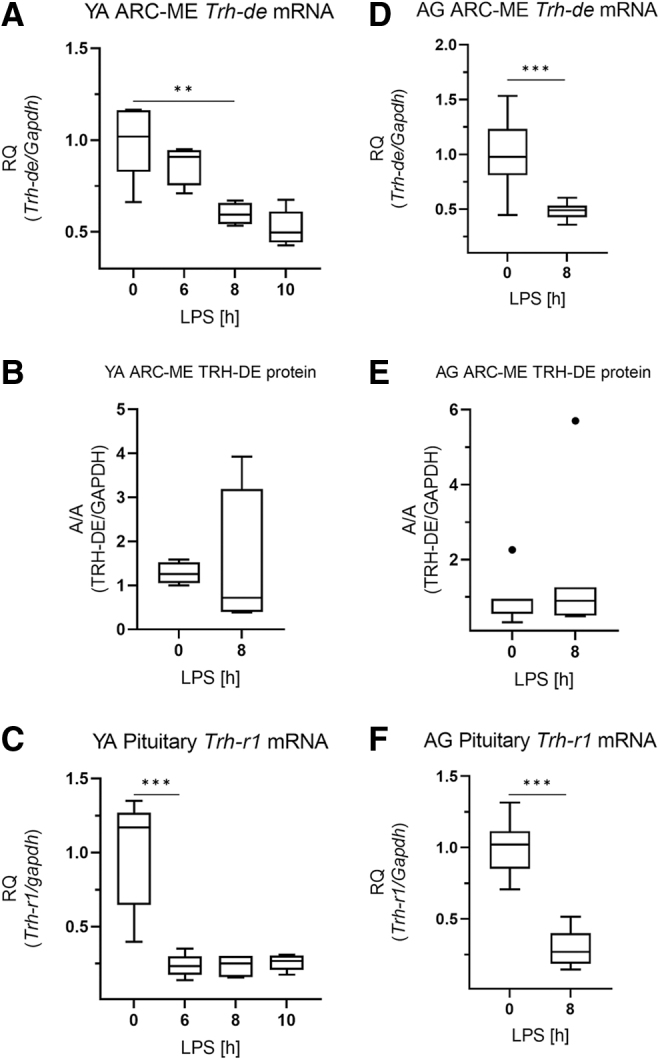
Regulators of TRH action in the HPT axis in LPS-induced NTIS. Male THAI mice were treated with 150 μg/animal LPS i.p. (A–C) YA (2–3 months old), (A) ARC-ME Trh-de mRNA levels, (B) ARC-ME TRH-DE protein levels, (C) pituitary Trh-r1 mRNA levels, (D–F) AG (1 year old), (D) ARC-ME Trh-de mRNA levels, (E) ARC-ME TRH-DE protein levels, (F) pituitary Trh-r1 mRNA levels. n(YA) = 5/group; n(AG) = 7–9/group; figure shows Tukey Box Plots, α = 0.05; **p < 0.01, ***p < 0.001. TRH, thyrotropin-releasing hormone; TRH-DE, thyrotropin-releasing hormone-degrading ectoenzyme.
However, this change was not reflected in the amount of TRH-DE protein in the ARC-ME region in neither age group, assessed by Capillary Microwestern (Fig. 5B, E; Supplementary Fig. S2). The expression of pituitary Trh-r1, the canonical receptor of TRH signaling in the pituitary, was markedly decreased after LPS-treatment, but no age-related differences were observed (Fig. 5C, F).
Coexistence of eu- and hypothyroidism in peripheral tissues after LPS-treatment
Hepatic Luciferase expression was not influenced by LPS-treatment in young adults, although hepatic Dio1 expression was markedly decreased by the treatment (Fig. 6A, B). In contrast, Luciferase expression of LPS treated mice was significantly decreased in the small intestine, representing reduced TH signaling 10 hours after the treatment (Fig. 6C). Similar changes were observed in aged animals (Fig. 6D–F).
FIG. 6.
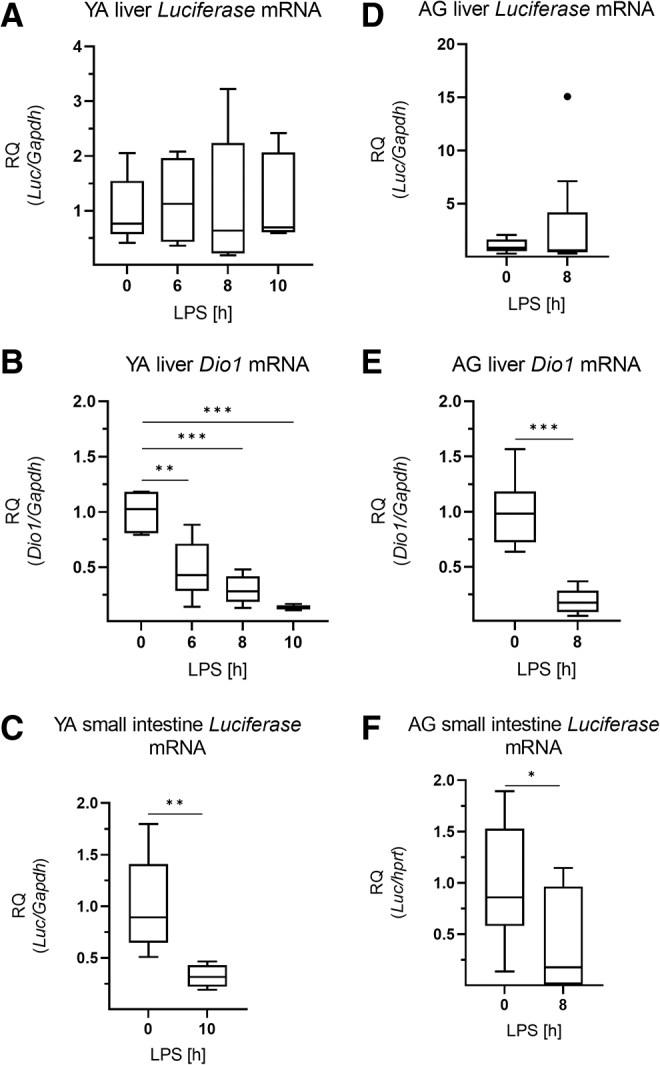
Local thyroid hormone action of peripheral tissues in LPS-induced NTIS. Male THAI mice were treated with 150 μg/animal LPS i.p.; Luciferase expression represents local TH-action. (A–C) YA (2–3 months old), (A) hepatic Luciferase mRNA levels, (B) hepatic Dio1 mRNA levels, (C) small intestine Luciferase mRNA levels, (D–F) AG (1 year old), (D) hepatic luciferase mRNA levels, (E) hepatic Dio1 mRNA levels, (F) small intestine Luciferase mRNA levels. n(YA) = 5/group; n(AG) = 7–9/group; figure shows Tukey Box Plots, α = 0.05; *p < 0.05, **p < 0.01, ***p < 0.001.
Response of the HPT axis during fasting is accompanied by unaltered hypothalamic TH signaling
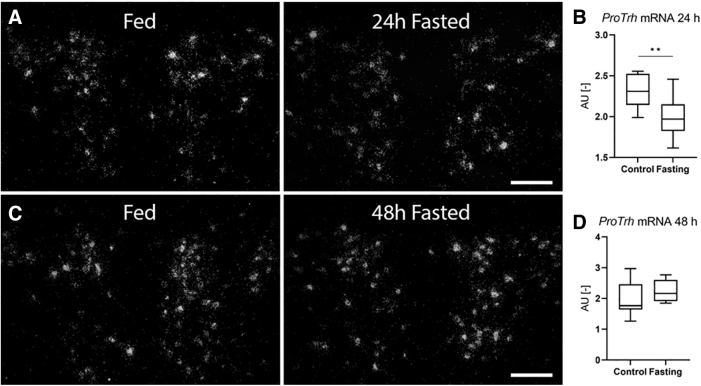
ProTrh expression was decreased by ∼15% in the PVN after 24 hours' fasting by in situ hybridization, while this decrease was already absent after 48 hours (Fig. 7A–D). Tshβ expression in the pituitary was decreased by both 24 and 48 hours' fasting (Fig. 8A, D). Serum fT3 but not fT4 was decreased after 24 hours (Fig. 8B, C), while both fT4 and fT3 were decreased after 48 hours' fasting (Fig. 8E, F). Fasting was accompanied by unchanged Luciferase expression in the ARC-ME region after both 24 and 48 hours' fasting, indicating unaltered hypothalamic TH signaling (Fig. 9A, C) while Luciferase expression was decreased in the pituitary (Fig. 9B, D). No changes of TH signaling were revealed that could be responsible for the response of the HPT axis during fasting, thus age-dependent studies under this condition were omitted.
FIG. 7.
proTrh expression in the PVN of fasting THAI mice. (A, B) 24 hours fasting, (C, D) 48 hours' fasting. (A, C) Representative dark-field micrographs of proTrh in situ hybridization at the mid-level PVN of fasting YA. (B) Quantification of the proTrh mRNA hybridization signal of (A), (D) Quantification of the proTrh mRNA hybridization signal of (C). n(24 hours) = 10–11/group; n(48 hours) = 7–8/group; figure shows Tukey Box Plots, α = 0.05; **p < 0.01. Scale bar: 100 μm.
FIG. 8.
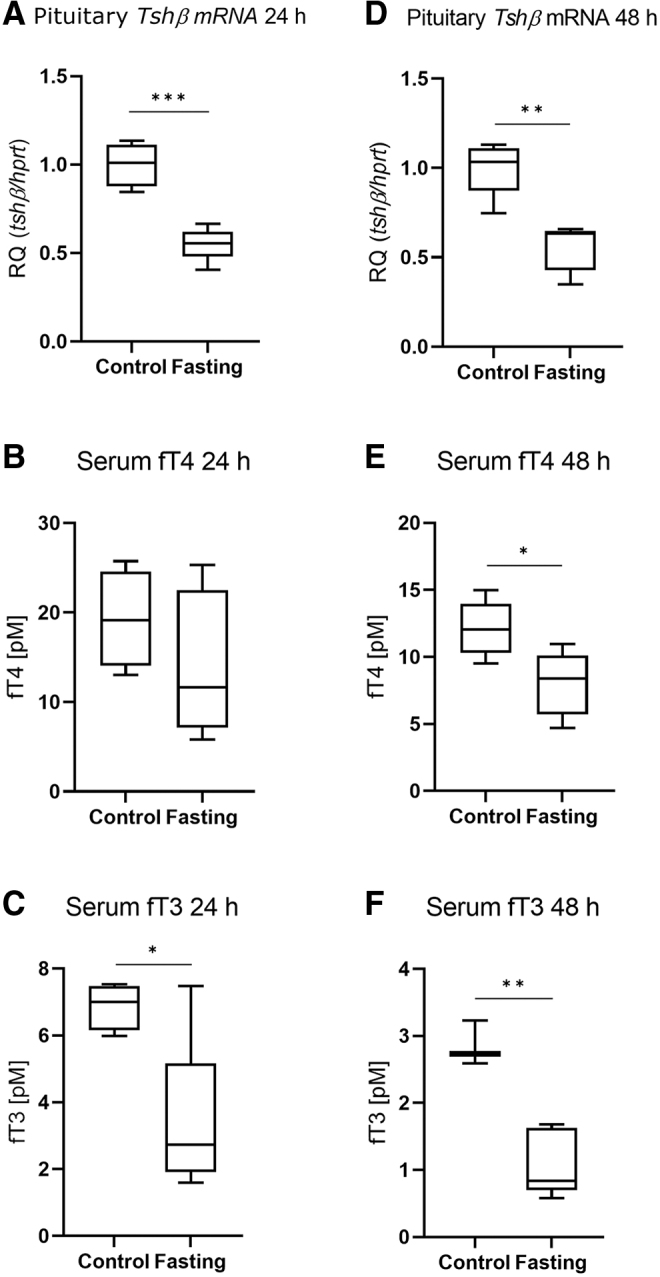
Responsiveness of the HPT axis in fasting animals. Male THAI mice were fasted for 24 and 48 hours. (A–C) 24 hours' fasting, (D–F) 48 hours' fasting, (A) pituitary Tshβ mRNA levels, (B) serum fT4 levels, (C) serum fT3 levels, (D) pituitary Tshβ mRNA levels, (E) serum fT4 levels, (F) serum fT3 levels. n(24 hours) = 4–5/group; n(48 hours) = 3–5/group; figure shows Tukey Box Plots, α = 0.05; *p < 0.05, **p < 0.01, ***p < 0.001.
FIG. 9.
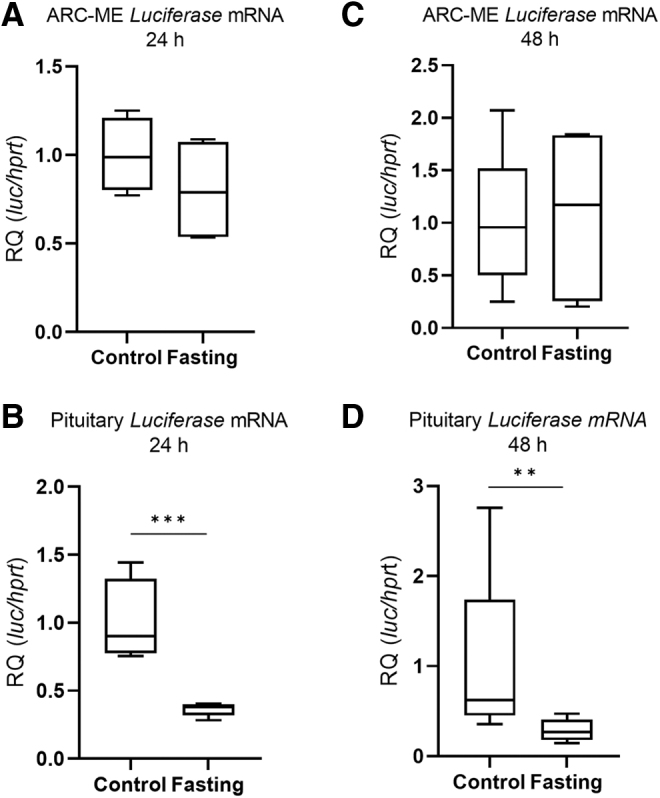
Thyroid Hormone action in the HPT axis in fasting animals. Male THAI mice were fasted for 24 and 48 hours, Luciferase expression represents local TH-action. (A, B) 24 hours' fasting, (C, D) 48 hours' fasting, (A) ARC-ME Luciferase mRNA levels, (B) pituitary Luciferase mRNA levels, (C) ARC-ME Luciferase mRNA levels, (D) pituitary Luciferase mRNA levels; n(24 hours) = 4–5/group; n(48 hours) = 5/group; figure shows Tukey Box Plots, α = 0.05; **p < 0.01, ***p < 0.001.
Discussion
The NTIS frequently occurs in hospitalized patients and is hallmarked by central inhibition of TRH secretion and by reduced serum TSH and TH levels, yet its pathogenesis is still only partially understood.1,16,17 In rodents, infection-induced NTIS can be mimicked by LPS-treatment. Earlier studies demonstrated that LPS-treatment also influences local TH metabolism in certain organs, suggesting both central and peripheral modulation of the TH-action.4,5,18
Data from our and other laboratories indicated that localized hypothalamic increase of D2 activity in tanycytes could play a role in the development of NTIS in rats and mice.4,5 While circulating TH levels has an ∼50% decrease 12 hours after LPS-administration, the D2 activity in the tanycytes increases fourfold.4 Thus, we suggested that the net effect of these changes could be local hyperthyroidism in the MBH that can modulate the feedback regulation of hypophysiotropic TRH neurons and, therefore, could contribute to the inhibition of the HPT axis by LPS-treatment.4 This hypothesis was also supported by the absence of LPS-induced inhibition of the HPT axis in D2 knockout mice.6 However, the concept was questioned by data reporting that T3 concentration in the homogenate of complete hypothalamic blocks was not influenced by LPS-treatment.8
Therefore, we took advantage of THAI mouse, a transgenic mouse model allowing the tissue-specific assessment of endogenous changes in local TH-action.10 THAI mice were treated with LPS and TH signaling was measured in the ARC-ME region where D2 expressing tanycytes and the axon terminals of the hypophysiotropic TRH neurons are located.19,20
Our data revealed increased TH-action in the ARC-ME region despite falling circulating TH levels. The increase of Luciferase expression in this hypothalamic region is preceded by the increase of D2 expression in tanycytes, further supporting that the LPS-induced increase of D2 activity in these cells can induce local hyperthyroidism in the neuropil surrounding the tanycytes. The ARC-ME region is critical in the feedback regulation of the hypophysiotropic TRH neurons.
The axon terminals of these cells terminate around the fenestrated capillaries of the external zone of the ME.21 As the ME is located outside of the blood–brain barrier, circulating T3 can freely enter into the extracellular space of the ME. The T3 content of this region, however, also depends on the T3 released from tanycytes after conversion of the prohormone T4 to the active T3. In the external zone of the ME, the end feet processes of tanycytes and the axon terminals of the hypophysiotropic TRH neurons are in close contact and MCT8 is also present on the surface of TRH axons.21
This anatomical relationship suggests that the T3 content of the ME is monitored by the hypophysiotropic TRH neurons and it plays a critical role in the negative feedback regulation of the TRH synthesis. The demonstrated local hyperthyroidism in the hypothalamic ARC-ME was followed by the decrease of proTrh expression in the PVN. This indicates that local increase of TH-action in the MBH plays a major role in impaired HPT activity.
This is also supported by fact that the TRH expression is decreased in the PVN at the same time when the Luciferase expression is increased in the ARC-ME region. Therefore, the LPS-induced local hyperthyroidism is likely sufficient to inhibit the hypophysiotropic TRH neurons, despite the fact that the increased D2 activity of tanycytes is not sufficient to increase the TH-action in the entire hypothalamus after LPS-treatment.
Intriguingly, TH-action is regulated differently in peripheral tissues. In contrast to the ARC-ME, pituitary TH-action was decreased by LPS that was a net result of lower circulating TH levels and the simultaneous decrease of D2 activity in this tissue. Since D2 activity is negatively regulated by TH availability,22 it is likely that the LPS itself or the LPS-induced cytokines could be responsible for decreased D2 activity of the pituitary.
The direct action of these substances is supported by the presence of cytokine and toll-like receptors in the pituitary.23,24 Surprisingly, D2 activity and Dio3 expressions changed parallel in the pituitary. As Dio3 expression is regulated positively by TH,22 it is likely that the decreased Dio3 expression represents a compensatory mechanism that tries to preserve pituitary TH-action to suppression of Tsh.
As a hallmark of NTIS, pituitary Tshβ expression was decreased as found earlier in LPS-injected rats and mice4,5 accompanied with decreased serum TSH. The revealed decrease in pituitary TH signaling is interesting, since Tshβ is negatively regulated by TH.20 This finding reveals that the decreased Tshβ expression in LPS-induced NTIS is not the consequence of local pituitary TH-action, but it is likely that the result of the decreased TRH expression and release overrides the stimulatory effect of local pituitary hypothyroidism on Tshβ expression.
Accumulating data support the hypothesis of a life-stage specific optimum of tissue TH signaling. Decreased TH levels have been associated with longer lifespan in animal models.15,25–27 In addition, human clinical studies revealed a negative correlation between longevity and circulating TH levels.28 We studied how tissue-specific TH-action is modulated by age during the challenge of LPS-induced NTIS.
Our findings on Tshβ and circulating fT4 of 1-year-old THAI mice demonstrated an age-evoked impaired responsiveness of the HPT axis that hindered to develop the significantly decreased Tshβ and circulating fT4 of young adults, characteristic hallmarks of NTIS. This age-dependent difference was not reflected in fT3 levels, which is not surprising since peripheral TH metabolism plays an important role in the regulation of the circulating fT3 levels.22 Although proTrh expression in the PVN decreased similarly in aged and in young adults, this change could not be translated in aged animals neither to decreased Tshβ expression nor to the fall of circulating fT4 levels.
We speculated whether this is caused by an elevated basal proTrh mRNA expression in the PVN of aged animals; thus, a similar level of LPS-induced reduction in proTrh expression could result in higher Tshβ levels in aged animals. Since situ hybridization did not reveal an age-dependent difference in proTrh expression in the PVN, we rejected this idea. Age-dependent differences in local pituitary TH-action were absent, therefore we looked for age-dependent changes of modulators of TRH levels.
TRH-DE (or pyroglutamil peptidase II) is an exocellular deactivator of the TRH peptide and is highly expressed by the tanycytes29 but LPS-treatment did not change TRH-DE protein level in the MBH. In addition to the lower proTrh mRNA levels in the PVN, the decreased expression of pituitary Trh-r1 also points toward a suppressed TRH signal on thyrotrophic cells in the pituitary after LPS-treatment. As the decrease of Trh-r1 expression was not age-dependent, it is likely that the age-dependent regulation of TSH expression is not due to age-dependent regulation of TRH release, but rather an intrinsic feature of the thyrotropes.
The set-point of the HPT axis that determines the aimed range of circulating TH levels is pre-set around birth and remains unchanged during the entire lifespan.20 Since a different optimum of TH homeostasis is suggested for specific life periods, the fixed set-point represents a potential bottleneck in deregulation of TH homeostasis that could lead to tissue-dysfunctions during aging. Our data demonstrate that under specific conditions, responsiveness of the HPT axis undergoes age-dependent changes despite the fixed set-point.
In addition, NTIS also generated an age-independent but a tissue-specific pattern of TH-action in peripheral organs. Our finding on unchanged hepatic TH-action up to 10 hours after LPS injection differs from data showing a ∼20% decreased hepatic T3 content in LPS-treated female mice at this time point.18 It is unclear why this decrease was not reflected in hepatic TH-action. Since our studies were performed in males, sex- or strain-specific effects cannot be excluded, but modulation of TH transport or the TH receptor complex could be also the reason for this effect.
In addition, our finding on unaltered hepatic TH-action also underlines that the decrease of Dio1, a known TH-regulated marker gene in the liver, is not the result of decreased TH signaling, but likely a direct effect of cytokines that were shown to interfere with Dio1 expression.30 This further substantiates that endogenous TH-responsive genes face serious limitations when used to monitor changes of tissue-specific TH-action.
In contrast to LPS-induced NTIS, fasting was not associated with altered hypothalamic TH signaling although proTrh expression was decreased in the PVN after 24 hours' fasting accompanied with decreased circulating TH levels and pituitary Tshβ expression. In rats, proTrh expression is robustly decreased in the PVN,7 while available data on mice are limited and controversial.31–34 Our studies based on repeated experiments on 24 and 48 hours' fasted mice pointed out that the decrease of proTrh expression in the PVN of mice is moderate compared with that of rats.
We also ran pilot studies with similar results on fasting C57Bl/6 mice that indicated that modest response of proTrh expression in the PVN of fasting mice is not specific for the used FBV/Ant background. Our findings demonstrate that in contrast to LPS-induced NTIS, the hypothalamic regulation of proTrh expression in fasted mice is not associated with a signature of elevated TH signaling in the MBH. Decreased pituitary TH-action was found in both LPS and fasting-induced NTIS.
Although this could indicate that TSHβ is not regulated dominantly by local TH-action in the pituitary in either condition, it should be also considered that thyrotrophs represent only a subpopulation of the pituitary and our measurement is the net result of the full anterior pituitary, thus the thyrotroph-specific conditions are unknown.
Our data also demonstrate that THAI mice can reveal local tissue-specific changes of TH-action independently from circulating TH levels. These findings strongly call for the development of approaches to allow assessment of tissue TH-action in patients.
Supplementary Material
Acknowledgments
The authors thank Andrea Juhász for her excellent technical support.
Authors' Contributions
R.S.: Methodology (lead), writing—review and editing. P.M., D.K., V.P., G.W., L.M., and T.L.F.: Methodology. A.C.B.: Methodology, writing—original draft (supporting). C.F. and B.G.: Methodology, conceptualization, writing—review and editing.
Author Disclosure Statement
A.C.B. is a consultant for AbbVie, Synthonics, Sention, and Thyron. R.S., P.M., D.K., V.P., G.W., L.M., T.L.F., C.F., and B.G. have nothing to disclose.
Funding Information
This work was supported by the National Research, Development and Innovation Office (NKFIH) of Hungary Grants K125247 (to B.G.), K138487 (to C.F.), the Hungarian National Brain Research Program 2017-1.2.1-NKP-2017-00002 (to C.F. and B.G.), and NIH 2R01DK058538-14A1 (to A.C.B. and B.G.). R.S., P.M., D.K., V.P., G.W., L.M., and T.L.F. have nothing to report.
Supplementary Material
References
- 1. Boelen A, Kwakkel J, Fliers E. Beyond low plasma T3: Local thyroid hormone metabolism during inflammation and infection. Endocr Rev 2011;32(5):670–693; doi: 10.1210/er.2011-0007 [DOI] [PubMed] [Google Scholar]
- 2. De Groot LJ. Dangerous dogmas in medicine: The nonthyroidal illness syndrome. J Clin Endocrinol Metab 1999;84(1):151–164; doi: 10.1210/jcem.84.1.5364 [DOI] [PubMed] [Google Scholar]
- 3. Van den Berghe G. Non-thyroidal illness in the ICU: A syndrome with different faces. Thyroid 2014;24(10):1456–1465; doi: 10.1089/thy.2014.0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fekete C, Gereben B, Doleschall M, et al. Lipopolysaccharide induces type 2 iodothyronine deiodinase in the mediobasal hypothalamus: Implications for the nonthyroidal illness syndrome. Endocrinology 2004;145(4):1649–1655. [DOI] [PubMed] [Google Scholar]
- 5. Boelen A, Kwakkel J, Thijssen-Timmer DC, et al. Simultaneous changes in central and peripheral components of the hypothalamus-pituitary-thyroid axis in lipopolysaccharide-induced acute illness in mice. J Endocrinol 2004;182(2):315–323. [DOI] [PubMed] [Google Scholar]
- 6. Freitas BC, Gereben B, Castillo M, et al. . Paracrine signaling by glial cell-derived triiodothyronine activates neuronal gene expression in the rodent brain and human cells. J Clin Invest 2010;120(6):2206–2217; doi: 10.1172/JCI41977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Legradi G, Emerson CH, Ahima RS, et al. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology 1997;138(6):2569–2576. [DOI] [PubMed] [Google Scholar]
- 8. Mebis L, Debaveye Y, Ellger B, et al. . Changes in the central component of the hypothalamus-pituitary-thyroid axis in a rabbit model of prolonged critical illness. Crit Care 2009;13(5):R147; doi: 10.1186/cc8043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Diano S, Naftolin F, Goglia F, et al. Fasting-induced increase in type II iodothyronine deiodinase activity and messenger ribonucleic acid levels is not reversed by thyroxine in the rat hypothalamus. Endocrinology 1998;139(6):2879–2884. [DOI] [PubMed] [Google Scholar]
- 10. Mohacsik P, Erdelyi F, Baranyi M, et al. . A transgenic mouse model for detection of tissue-specific thyroid hormone action. Endocrinology 2018;159(2):1159–1171; doi: 10.1210/en.2017-00582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palkovits M. Microdissection of individual brain nuclei and areas. In: Neuromethods. (Boulton AA, Baker GB. eds.) Humana Press: Totowa, NJ, USA; 1986; pp. 1–17. [Google Scholar]
- 12. Fekete C, Kelly J, Mihaly E, et al. . Neuropeptide Y has a central inhibitory action on the hypothalamic-pituitary-thyroid axis. Endocrinology 2001;142(6):2606–2613; doi: 10.1210/endo.142.6.8207 [DOI] [PubMed] [Google Scholar]
- 13. Bianco AC, Anderson G, Forrest D, et al. . American thyroid association guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid 2014;24(1):88–168; doi: 10.1089/thy.2013.0109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fekete C, Sarkar S, Christoffolete MA, et al. Bacterial lipopolysaccharide (LPS)-induced type 2 iodothyronine deiodinase (D2) activation in the mediobasal hypothalamus (MBH) is independent of the LPS-induced fall in serum thyroid hormone levels. Brain Res 2005;1056(1):97–99. [DOI] [PubMed] [Google Scholar]
- 15. Bowers J, Terrien J, Clerget-Froidevaux MS, et al. . Thyroid hormone signaling and homeostasis during aging. Endocr Rev 2013;34(4):556–589; doi: 10.1210/er.2012-1056 [DOI] [PubMed] [Google Scholar]
- 16. Warner MH, Beckett GJ. Mechanisms behind the non-thyroidal illness syndrome: An update. J Endocrinol 2010;205(1):1–13; doi: 10.1677/JOE-09-0412 [DOI] [PubMed] [Google Scholar]
- 17. Mebis L, Van den Berghe G. Thyroid axis function and dysfunction in critical illness. Best Pract Res Clin Endocrinol Metab 2011;25(5):745–757; doi: 10.1016/j.beem.2011.03.002 [DOI] [PubMed] [Google Scholar]
- 18. Boelen A, van der Spek AH, Bloise F, et al. . Tissue thyroid hormone metabolism is differentially regulated during illness in mice. J Endocrinol 2017;233(1):25–36; doi: 10.1530/JOE-16-0483 [DOI] [PubMed] [Google Scholar]
- 19. Gereben B, McAninch EA, Ribeiro MO, et al. . Scope and limitations of iodothyronine deiodinases in hypothyroidism. Nat Rev Endocrinol 2015;11(11):642–652; doi: 10.1038/nrendo.2015.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev 2014;35(2):159–194; doi: 10.1210/er.2013-1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kallo I, Mohacsik P, Vida B, et al. . A novel pathway regulates thyroid hormone availability in rat and human hypothalamic neurosecretory neurons. PLoS One 2012;7(6):e37860; doi: 10.1371/journal.pone.0037860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gereben B, Zeold A, Dentice M, et al. . Activation and inactivation of thyroid hormone by deiodinases: Local action with general consequences. Cell Mol Life Sci 2008;65(4):570–590; doi: 10.1007/s00018-007-7396-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Breuel KF, Kougias P, Rice PJ, et al. . Anterior pituitary cells express pattern recognition receptors for fungal glucans: Implications for neuroendocrine immune involvement in response to fungal infections. Neuroimmunomodulation 2004;11(1):1–9; doi: 10.1159/000072963 [DOI] [PubMed] [Google Scholar]
- 24. Haedo MR, Gerez J, Fuertes M, et al. . Regulation of pituitary function by cytokines. Horm Res 2009;72(5):266–274; doi: 10.1159/000245928 [DOI] [PubMed] [Google Scholar]
- 25. Brown-Borg HM. Hormonal regulation of longevity in mammals. Ageing Res Rev 2007;6(1):28–45; doi: 10.1016/j.arr.2007.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Buffenstein R, Pinto M. Endocrine function in naturally long-living small mammals. Mol Cell Endocrinol 2009;299(1):101–111; doi: 10.1016/j.mce.2008.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dell'agnello C, Leo S, Agostino A, et al. . Increased longevity and refractoriness to Ca(2+)-dependent neurodegeneration in Surf1 knockout mice. Hum Mol Genet 2007;16(4):431–444; doi: 10.1093/hmg/ddl477 [DOI] [PubMed] [Google Scholar]
- 28. Jansen SW, Akintola AA, Roelfsema F, et al. Human longevity is characterised by high thyroid stimulating hormone secretion without altered energy metabolism. Sci Rep 2015;5:11525; doi: 10.1038/srep11525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sanchez E, Vargas MA, Singru PS, et al. . Tanycyte pyroglutamyl peptidase II contributes to regulation of the hypothalamic-pituitary-thyroid axis through glial-axonal associations in the median eminence. Endocrinology 2009;150(5):2283–2291; doi: 10.1210/en.2008-1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yu J, Koenig RJ. Regulation of hepatocyte thyroxine 5'-deiodinase by T3 and nuclear receptor coactivators as a model of the sick euthyroid syndrome. J Biol Chem 2000;275(49):38296–38301. [DOI] [PubMed] [Google Scholar]
- 31. Abel ED, Ahima RS, Boers ME, et al. . Critical role for thyroid hormone receptor beta2 in the regulation of paraventricular thyrotropin-releasing hormone neurons. J Clin Invest 2001;107(8):1017–1023; doi: 10.1172/JCI10858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vella KR, Ramadoss P, Lam FS, et al. . NPY and MC4R signaling regulate thyroid hormone levels during fasting through both central and peripheral pathways. Cell Metab 2011;14(6):780–790; doi: 10.1016/j.cmet.2011.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Minakhina S, De Oliveira V, Kim SY, et al. . Thyroid hormone receptor phosphorylation regulates acute fasting-induced suppression of the hypothalamic-pituitary-thyroid axis. Proc Natl Acad Sci U S A 2021;118(39):e2107943118; doi: 10.1073/pnas.2107943118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Campos AMP, Wasinski F, Klein MO, et al. Fasting reduces the number of TRH immunoreactive neurons in the hypothalamic paraventricular nucleus of male rats, but not in mice. Neurosci Lett 2021;752:135832; doi: 10.1016/j.neulet.2021.135832 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.