Abstract
Objective:
To conduct a systematic review and meta-analysis of recently published randomized controlled trials (RCTs) that employed the use of topical oxygen therapy (TOT) as an adjunct therapy in the treatment of Wagner 1 and 2 diabetic foot ulcers.
Approach:
Following a literature search of eligible studies from 2010 onward, four RCTs were included. Studies were analyzed for patient and wound characteristics, outcomes, risk of bias, and quality of the evidence assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology. A random-effects meta-analysis for complete wound healing was carried out due to statistical heterogeneity of included studies.
Results:
Risk of bias judgment (RoB2 analysis) resulted in one low-risk trial and three trials with some risk. One study was determined to be the origin of the statistical heterogeneity. Pooled results showed statistical significance with a risk ratio (RR) of 1.59 (95% confidence interval [CI]: 1.07–2.37; p = 0.021). Sensitivity analysis, based on imputed values for missing outcomes, demonstrated that both the RR and 95% CIs changed little. The GRADE ratings for each domain were as follows: (a) risk of bias: moderate (3); (b) imprecision: moderate (2), high (1); (c) inconsistency: low (2), high (1); (d) indirectness: moderate (2), high (1); and (e) publication bias: moderate (1), high (2). Overall, the evidence was moderate.
Innovation:
Our study shows that TOT is a viable diabetic foot ulcer therapy.
Conclusions:
These data support the use of TOT for the treatment of chronic Wagner 1 or 2 diabetic foot ulcers in the absence of infection and ischemia.
Keywords: diabetic foot ulcer, topical oxygen therapy, systematic review, meta-analysis

Marissa J. Carter, PhD, MA
INTRODUCTION
Diabetes-related foot ulcers (DFUs), affecting up to one third of patients with diabetes during the course of their disease, have long been considered major precursors to lower extremity amputation, whether of neuropathic, ischemic, or neuroischemic etiology.1,2 A recent systematic review reported a worldwide prevalence of DFU of 6.3%, with the highest prevalence being 13% in North America.3 Another report found the global burden of diabetes-related lower extremity complications to be high, with a crude estimate of DFUs affecting 18.6 million persons.4 The costs of treating chronic DFUs are commensurate with the frequent complexity of the problem itself. A 2014 U.S.-based administrative database study found that such costs can range from $9 to $13 billion, and the excess health care costs of DFU approximately double the cost of treating diabetes itself.5
The rapid growth of the global population with diabetes will lead to a greater number of patients suffering from DFUs and a concomitant increase in global amputation rates. Current protocols for managing acute or chronic DFUs focus on thorough systematic assessment, debridement, effective offloading, wound bed preparation, and revascularization, as necessary.6–8 Unfortunately, many DFUs are refractory to optimal standard of care (SOC) as defined by healing less than 50% in 4 weeks. These patients require reassessment of vascular, nutritional, and infection status; examination of the effectiveness of debridement and offloading regimens; and reevaluation of renal status, diabetic control, residual small artery disease (not amenable to intervention), or other significant metabolic abnormalities that may adversely affect wound healing. These patients are likely to benefit from advanced wound healing therapies, such as topical oxygen therapy (TOT).7,9,10
When TOT was introduced to clinical practice over 50 years ago, practitioners initially viewed it as a controversial therapy, due to the sparse evidence supporting its use. Technological advances in delivery systems and recent clinical trials suggest that TOT is a viable advanced treatment modality for DFUs.10–15
There are several types of TOT devices on the market, including continuous diffusion of oxygen (CDO) delivery systems, devices delivering low constant pressure O2 (at 22 mmHg), and a system that delivers cyclically pressurized oxygen (from 10 to 50 mb above atmospheric pressure).10,16 CDO devices, delivering a low constant flow of pure oxygen at 3–15 mL/h, are designed for continuous ambulatory use through proprietary wound dressings, whereas the latter two devices deliver oxygen within a flexible, disposable extremity chamber used in the home.
Early clinical studies on TOT lacked scientific rigor. For example, a poorly designed, unblinded, and inadequately powered trial published in 1988 compared 12 hospitalized patients with chronic DFUs who received 14 days of topical “hyperbaric” oxygen therapy with 16 similar patients treated with standard care. The investigators reported no difference in healing outcomes after 2 weeks of treatment, and they concluded that TOT provided no appreciable benefit in the treatment of DFUs.17
Despite this early report, several reviews, observational human studies, and preclinical animal work suggested a positive wound healing benefit associated with TOT.18–24 A very compelling animal study was published by Fries et al. in 2017 that clearly demonstrated an upregulation of growth factors and significant increases in tissue level oxygen partial pressures after treatment with TOT compared with control wounds.19 Furthermore, these physiological attributes were corroborated by clinical as well as histological evidence of improved healing in the oxygen-treated wounds.
The last decade, however, has given rise to several more robust randomized controlled trials (RCTs) that demonstrate the efficacy of TOT in treatment of chronic DFUs compared with controls treated with SOC alone.11–15 Additionally, several recent independent systematic reviews and meta-analyses validate the additional benefit of TOT in this regard.25–27 These systematic reviews, however, were fairly heterogeneous in the types of studies analyzed and did not include the most recent RCT published in 2021.13
Our aim in this systematic review and meta-analysis was to provide a rigorous assessment of recently reported RCTs comparing adjunctive TOT with control patients receiving SOC for the treatment of chronic DFUs. Distinct from the several other systematic reviews, we include only robust prospective RCTs with primary outcomes of complete healing at 12 weeks. Complete healing (or complete wound closure), as used in this analysis, is defined as complete reepithelialization with no further evidence of drainage nor visible granulation tissue. To avoid potential duplication of patient data, we have specifically excluded those publications with reported interim data. The data from studies evaluating CDO and cyclically pressurized systems were included and pooled. There were too few studies to perform separate meta-analyses.
INNOVATION
TOT has had a checkered history as an adjunctive therapy in wound healing in part due to the development of the technology itself. Recent advances have made the devices more robust and reliable with the potential to accelerate wound healing in chronic diabetic foot ulcers. By conducting a systematic review of all the relevant RCTs using the most conservative outcome of complete wound healing at a minimum of 12 weeks, we demonstrate that more robust devices do have the ability to improve wound healing in less severe chronic diabetic foot ulcers.
CLINICAL PROBLEM ADDRESSED
Chronic diabetic foot ulcers are an unfortunate but common complication of diabetes. While consistent SOC can heal many DFUs given enough time, a substantial proportion of these wound types are stuck in the inflammatory phase of wound healing and need an adjunctive treatment to address the issue(s) so that the wound can transition to the proliferative phase. While a variety of treatments have demonstrated efficacy in this regard, many are expensive, and some are not available to all patients. Given that TOT is a possible adjunctive therapy for DFUs, we decided to formally assess the RCTs conducted to date using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE), including meta-analysis, to determine if this mode of therapy can be recommended.
MATERIALS AND METHODS
The study was performed according to the 2015 Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) statement28 and was registered at PROSPERO (CRD42021259090).
Eligibility
Studies involving patients with chronic DFUs using TOT (intermittent or continuous application of oxygen) in addition to SOC (debridement, offloading, and moist wound care) in any health care setting were eligible provided that they were RCTs. Data of studies with 20 patients or fewer or without primary outcomes of healing at 12 weeks were excluded from the assessment.
Literature search
The following databases were used in the search:
PubMed
MEDLINE/OVID
Embase
Cochrane Central Register of Controlled Trials
Cochrane Database of Systematic Reviews
Health Technology Assessment Database
ClinicalTrials.gov
International Clinical Trials Registry Platform Search Portal.
The following search terms were used: Topical Oxygen OR Topical Wound Oxygen OR Continuous Diffusion of Oxygen OR Continuous Topical Oxygen OR Topical Hyperbaric Oxygen OR High-Pressure Cyclical Oxygen AND Clinical Trial OR Trial OR Placebo OR Random.
In addition, the full-text document (e.g., journal article or clinical study report) had to be available and published after January 1, 2010. Any language was permissible provided that English titles and abstracts were available and indicated potential relevance.
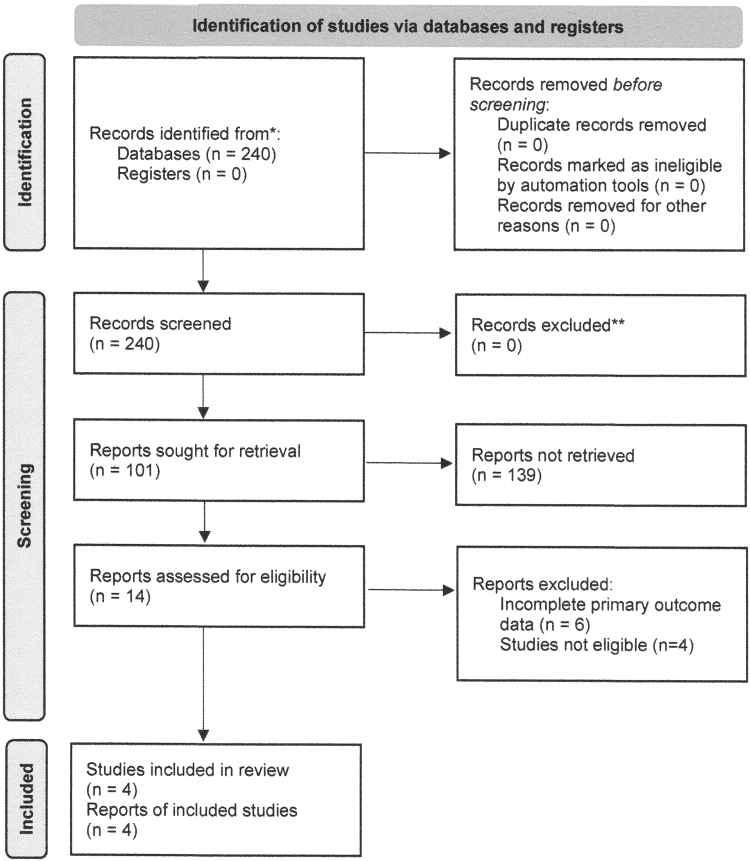
Figure 1 summarizes the literature search and study selection process. On October 20, 2021, 2 authors conducted the literature search, which yielded 51 clinical trials and 240 publications with 11 RCTs (14 publications) meeting search criteria (Table 1). All members of the study team discussed the search results and reached a consensus that four studies met the predefined inclusion criteria (Supplementary Tables S1 and S2).11–13,15
Figure 1.
PRISMA flow diagram. PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis.
Table 1.
Literature search data
| Parameter | Clinical Trials | Publications |
|---|---|---|
| Unique results from search terms | 51 | 240 |
| Those involving gaseous, topically applied oxygen | 30 | 101 |
| RCTs meeting criteria | 11 | 14 |
| Completed with full-text publication | 4 | 10a |
Two clinical trials had multiple publications. Only one reference from each study contained the primary outcomes used in the meta-analysis. Refer to Supplementary Tables S1 and S2.
RCT, randomized controlled trial.
Data extraction and risk of bias analysis
The primary outcome of interest was complete wound healing (skin reepithelialization without drainage or dressing requirements confirmed at two consecutive study visits 2 weeks or more apart) at 12 weeks with the following secondary outcomes collected if available: wound-related pain, readmission to the hospital, health-related quality of life, dependence on outside help or need for care, adherence to prescribed therapy, and adverse events (AEs), including amputation and mortality.
SOC in treatment of DFUs includes debridement as necessary (typically sharp debridement), offloading of plantar ulcers, appropriate dressings to maintain a moist wound care bed, and infection control. Reporting should include debridement type and frequency, as well as details of dressings and offloading. Although findings were not tabulated, we noted if details were missing.
Two reviewers independently extracted key study metrics and outcomes, and a third member checked congruity of the data. A consensus among the team members was reached that only complete wound healing was a useful outcome, as not all studies provided other wound healing outcomes.
The risk of bias analysis used the RoB2 approach developed by the Cochrane group.29 The same reviewers who extracted the data carried out the risk of bias analysis independently. The senior systematic reviewer/statistician adjudicated differences in assessment for each domain for each study and reviewed the overall results.
Meta-analysis
Meta-analysis was conducted for complete wound healing at 12 weeks using all eligible studies, as only one study was low risk. A fixed-effects model (inverse variance method; Mantel–Haenszel) was used when heterogeneity was judged nonsignificant, including the I2 and Cochran's Q metrics. A random-effects model (DerSimonian and Laird method) was used when heterogeneity was judged significant. Heterogeneity was explored by systematically omitting one of the included studies. The effect measure used was relative risk, and the analysis was conducted using MedCalc and SPSS (v 28.0).
The primary sensitivity analysis was carried out using a modified jump-to-reference (J2R) approach,30 in which subjects lost to follow-up or withdrawals due to other reasons for the complete wound healing outcome in the TOT group were assumed to have had the same proportional outcomes as the control/sham/SOC only group. Since outcomes for subjects withdrawn from the studies depend on the reason why the subjects were withdrawn, for serious adverse events (SAEs) or AEs in which the event was serious enough that a subject was withdrawn from the study, the event would likely have affected the wound healing trajectory in an adverse manner. For nonserious AEs or most other reasons for withdrawals, the wound healing trajectory would likely have been similar to the healing rate of the uncensored control/sham/SOC only group.
Thus, the algorithm for a secondary sensitivity analysis was implemented first for the control/sham/SOC only group as: (a) index wounds of subjects who had an SAE/AE that forced withdrawal or caused a voluntary withdrawal by the subject were scored as not healed; death was scored as not healed. If insufficient data were reported to enable a determination, then the algorithm assumed that this category was 0; (b) all other index wounds were scored as healed/not healed according to the J2R method, which uses complete case results. The adjusted healing rate thus obtained was then used as the J2R value for the TOT group. When calculating the number of subjects in a sensitivity analysis, if fractions were involved, numbers of subjects were rounded up or down to integers depending on whether the fraction was >0.5 or not.
Grading of Recommendations Assessment, Development, and Evaluation
Three team members evaluated independently the body of evidence using the GRADE methodology.31 Consensus among all authors determined recommendations.
RESULTS
Study details
Three of the four studies had larger and similar numbers of enrolled participants (N = 130–146), whereas the Frykberg RCT15 was smaller (N = 73) (Table 2). The mean wound age was comparable among all studies, although the Driver study11 enrolled smaller area wounds on average (Table 2). All RCTs had a study length of 12 weeks.
Table 2.
Characteristics of included studies
| Study | Year | RCT Study Design | No. of Subjects Randomized | Study Duration, Weeks | Setting | Intention-to-Treat Analysis | Wound Characteristics | Right-Censored Outcomes |
|---|---|---|---|---|---|---|---|---|
| Driver et al.11 | 2017 | • Subject blinded • Assessor blinded • 2 Groups |
130 • Activea: 66 • Shama: 64 |
12 | 22 Wound clinics (United States and Canada) | • Active: 65b • Sham: 63b |
• Class: UT 1A • Mean wound age: Active: 17.7 weeks; Sham: 14.9 weeks • Mean wound area: Active: 2.0 cm2; Sham: 2.3 cm2 |
• Active: 12 • SOC: 17 |
| Niederauer et al.12 | 2018 | • Subject blinded • Assessor blinded • 2 Groups |
146 • Active: 74 • Sham: 72 |
12 | 34 Wound clinics (United States) | • Active: 74 • Sham: 72 |
• Class: UT 1A • Mean wound age: Active: 18.8 weeks; Sham: 20.5 weeks • Mean wound area: Active: 3.5 cm2; Sham: 3.9 cm2 |
• Active: 22 • SOC: 19 |
| Frykberg et al.15 | 2020 | • Subject blinded • Assessor blinded • 2 Groups |
73 • Active: 36 • Sham: 37 |
12 | 17 Diabetic foot centers (United States, United Kingdom, France, Germany, and Luxembourg) | • Active: 36 • Sham: 37 |
• Class: UT 1A–D, 2A–D • Mean wound age: Active: 22.9 weeks; Sham: 24.9 weeks • Mean wound area: Active: 3.0 cm2; Sham: 3.2 cm2 |
• Active: 3 • SOC: 4 |
| Serena et al.13 | 2021 | • No blinding • 2 Groups |
145 • Active: 81 • SOC: 64 |
12 | 19 Wound clinics (United States) | • Active: 81 • SOC: 64 |
• Class: Wagner 1 or 2 • Mean wound age: Active: 24.5 weeks; SOC: 23.8 weeks • Mean wound area: Active: 2.9 cm2; SOC: 3.5 cm2 |
• Active: 15 • SOC: 12 |
Active group defined as the group allocated to the intervention; sham group defined as the group allocated to SOC alone using a sham device.
Subjects randomized and also received allocated treatment.
SOC, standard of care; UT, University of Texas.
The difference between intervention and SOC groups in terms of complete wound healing ranged from 5% to 27% (all rates had higher healing rates compared with the SOC group), but only the Driver trial reported statistically nonsignificant results (Table 3). AEs were similar in both groups for all studies, although the absolute rates varied considerably.
Table 3.
Complete wound healing outcomes and safety analysis of included studies
| Study | Year | Outcome Dataa | Safety Results | Healing Confirmation Visit |
|---|---|---|---|---|
| Driver et al.11 | 2017 | • Activeb: 35/65 (54%) • Shamb: 31/63 (49%) • p = 0.42 |
• SAEs: Active: 12; Sham: 13 ○ All unrelated/unlikely related to device • AEs: Active: 53 Sham: 55 ○ 1 Probably related to SOC ○ 3 Possibly related to device • Infections: Active: 3; Sham: 10 |
2 Weeks after initial healing date |
| Niederauer et al.12 | 2018 | • Active: 24/74 (32%) • Sham: 12/72 (17%) • p = 0.027 |
• AEs: Active: 11; Sham: 13 ○ Related to study wound: Active: 6; Sham: 10 |
Follow-up telephone call/12-week durability visit |
| Frykberg et al.15 | 2020 | • Active: 15/36 (41%) • Sham: 5/37 (14%) • p = 0.007 |
• SAEs: Active: 10; Sham: 10 • AEs: Active: 8; Sham: 8 • Index limb amputations: Active: 2; Sham: 3 |
2 Weeks after initial healing date |
| Serena et al.13 | 2021 | • Active: 36/81 (44%) • SOC: 18/64 (28%) • p = 0.04 |
• AEs: Active: 41; SOC: 32 • Severe/life-threatening AEs: Active: 7; SOC: 8 • Possibly/probably related to product: Active: 1; SOC: 12 AEs |
2-Week confirmation visit (T.E. Serena, pers. comm.) |
Based on proportions of wounds healed at 12 weeks.
Active group defined as the group allocated to the intervention; sham group defined as the group allocated to SOC alone using a sham device.
AE, adverse event; SAE, serious adverse event.
Risk of bias analysis
There were eight disagreements between the two reviewers regarding judgment of the five domains for the four studies, half of which were for the Driver study.11 There was also a disagreement about the overall judgment for the Driver trial.11 After adjudication, this resulted in one low-risk trial and three trials with some risk (Table 4).
Table 4.
Final summary table risk of bias judgment for complete wound healing outcome at 12 weeks
| Study | Domain 1 | Domain 2 | Domain 3 | Domain 4 | Domain 5 | Overall |
|---|---|---|---|---|---|---|
| Driver et al.11 | Low risk | Low risk | Some concerns | Low risk | Low risk | Some concerns |
| Frykberg et al.15 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Niederauer et al.12 | Some concerns | Low risk | Some concerns | Low risk | Low risk | Some concerns |
| Serena et al.13 | Low risk | Some concerns | Some concerns | Low risk | Low risk | Some concerns |
Meta-analysis
Since only one study was low risk, meta-analysis proceeded with all eligible studies. A random-effects model was chosen because there was substantial heterogeneity (I2: 55.7%; p = 0.081). The simple explanation for the origin of the heterogeneity was the Driver study,11 as its removal resulted in no heterogeneity.
The pooled results showed statistical significance with a risk ratio (RR) of 1.59 (95% confidence interval [CI]: 1.07–2.37; p = 0.021) (Fig. 2 and Table 5). A funnel plot suggested that the magnitude of the intervention response varied considerably with the degree of precision (Fig. 3).
Figure 2.
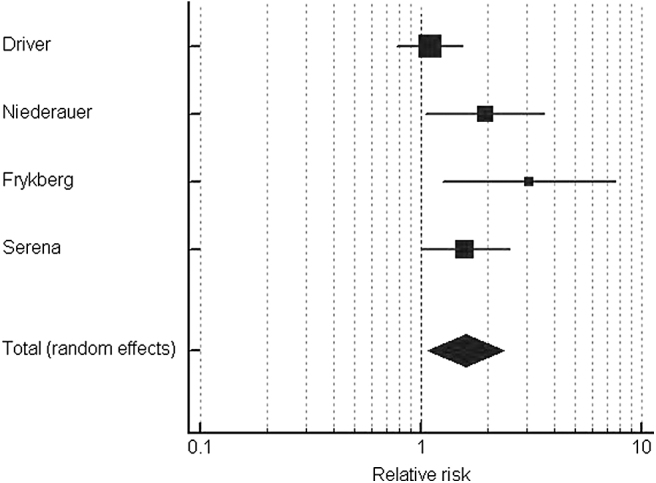
Forest plot for the random-effects model including all studies.
Table 5.
Results of the random-effects model including all studies
| Study | TOT | SOC | Risk Ratio | 95% CI | Weight, % |
|---|---|---|---|---|---|
| Driver et al.11 | 35/65 | 31/63 | 1.09 | 0.78–1.53 | 35.2 |
| Niederauer et al.12 | 24/74 | 12/72 | 1.95 | 1.06–3.59 | 22.2 |
| Frykberg et al.15 | 15/36 | 5/37 | 3.08 | 1.25–7.60 | 13.7 |
| Serena et al.13 | 36/81 | 18/64 | 1.58 | 1.00–2.51 | 28.8 |
| Total | 110/256 | 66/236 | 1.59 | 1.07–2.37 | 100.0 |
CI, confidence interval; TOT, topical oxygen therapy.
Figure 3.
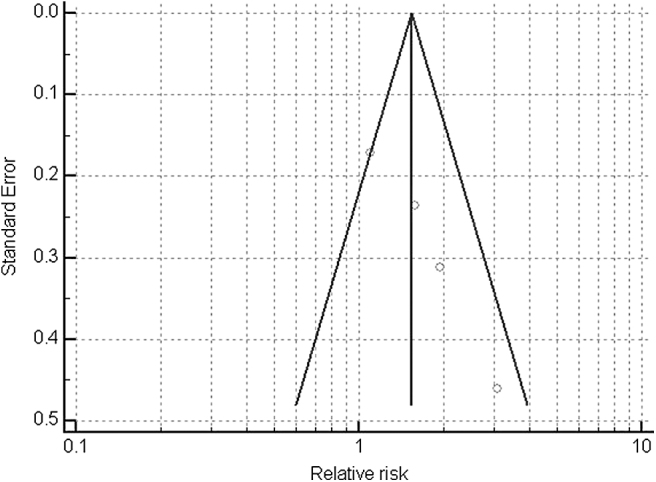
Funnel plot of the magnitude of the intervention response including all studies.
Sensitivity analysis using imputed values for missing outcomes, which was based on random-effects models, demonstrated that despite some fairly high levels of missing outcomes in three studies, neither the pooled estimate or 95% CIs changed substantially (Table 6) (p = 0.039).
Table 6.
Sensitivity analysis of the complete wound healing outcome using a random-effects model in the meta-analysis (p = 0.039)
Grading of Recommendations Assessment, Development, and Evaluation
The ratings for each domain were as follows: (a) risk of bias: moderate (3); (b) imprecision: moderate (2), high (1); (c) inconsistency: low (2), high (1); (d) indirectness: moderate (2), high (1); and (e) publication bias: moderate (1), high (2). All agreed that the overall evidence was moderate. Based on our meta-analysis and GRADE review, the study panel agreed that TOT can be recommended for the indication of chronic DFUs that have not responded to an initial trial of effective offloading and sharp debridement in the absence of infection and ischemia.
DISCUSSION
Our systematic review of RCT evidence for TOT indicates that it is a viable treatment for Wagner 1 and 2 DFUs. The meta-analysis supports efficacy in the defined populations under study; that is, chronic nonischemic DFUs of at least 4 weeks duration and not adequately responding to SOC alone. Our recommendation corroborates and is consistent with the other aforementioned systematic reviews that also support TOT for the treatment of chronic DFUs.25–27 Additional studies are needed to clarify the indications for use and efficacy in other wound types.
The meta-analysis demonstrated that, even in the presence of statistical heterogeneity addressed using random-effects models, results were statistically significant and remained so when applying reasonable sensitivity analysis. While other systematic reviews have already reported positive findings,25–27 our study was the first to analyze only RCTs, including the most recent trial,13 and we applied the most rigorous form of risk of bias analysis, GRADE, and meta-analysis.
It is interesting to note that if the Driver study11 is removed from meta-analysis, there is no heterogeneity, and a fixed-effects model is possible with extremely statistically significant results. One possible reason for the failure to achieve statistical significance in the Driver study11 could be premature failure of the active device, Epiflo™ (Neogenix, formerly Ogenix, Beachwood, OH). This older continuous topical oxygen (CTO) device uses an electrochemical generator to produce oxygen at a flow rate of 3 mL/h. While the device has a blinking red light that indicates when the power is on, it does not contain an alarm or indicator to notify the patient or provider that the generator is not functioning properly. If the device fails, the patient and clinician may believe that the device is working properly, even though it is no longer generating oxygen. In addition, CTO devices may not function in regions with low humidity levels.32
These weaknesses may explain the poorer results in the CTO clinical trials compared with the other systems. While the trial was generally well designed, the control group achieved nearly a 50% closure rate at 12 weeks compared with 54% in the active group. This would ostensibly indicate that those ulcers enrolled would have been as likely to heal with SOC as with active therapy. Furthermore, the authors acknowledged that they specifically excluded patients with typical comorbidities seen in patients with DFU (ischemia, renal insufficiency, etc.) and patients with deeper ulcers that would be considered more difficult to heal.11
The current SARS-Cov-2 pandemic has resulted considerable disruption to wound care especially for patients particularly for treatments that involve wound care clinics and hospitals.33 Consequently, treatments that can be conducted in the patient's home care setting may have some advantage,34 including TOT for treatment of DFUs. It remains unknown whether the economics of using TOT are favorable since no studies have been published on the subject. Although any advanced therapy will be more costly initially, long-term benefits are almost uniformly supportive of such therapies since they heal wounds faster and avoid hospitalizations and amputations. Indeed, a recent publication supports this premise.35
There are some limitations to this meta-analysis. First, complete wound healing was the only common endpoint available for this review and meta-analysis. The U.S. Food and Drug Administration favors this endpoint36; however, the analysis was unable to assess patient-centered outcomes, such as quality of life or wound-associated pain, or clinical endpoints of interest, such as prevention of infection or amputation. Second, there is invariably some heterogeneity in applying SOC for DFU treatment even in RCTs in which there is considerable training and assessment at each participating site; examples include types of dressings, debridement frequencies, and types of permissible offloading. Within a trial, heterogeneity is usually minimal due to randomization procedures, but between trials, differences can be a factor in determining what the overall SOC healing rate is over the length of the study.
However, what really matters is the difference between the treatment groups in regard to healing rates rather than absolute values. SOC can also be insufficiently detailed in the study publication, which is a reporting issue; for example, only two of the trials described debridement, and offloading details were missing in one trial. This can lead to uncertainty in regard to study assessment, particularly for assessors not familiar with the trials. Third, our recommendations are limited to Wagner grade 1 or 2 DFUs: none of the clinical trials included more severe ulcers. Similarly, the trials primarily treated chronic uninfected ulcers with adequate perfusion. Therefore, the conclusions do not extend to infected or ischemic ulcers. Last, our recommendations are based on only four RCTs; more well-conducted trials would be likely to strengthen or amend our conclusions.
In conclusion, this systemic review and meta-analysis supports the use of TOT as an adjunctive therapy in the treatment of Wagner 1 and 2 DFUs that have not responded to preliminary treatment with optimal SOC alone.
KEY FINDINGS
TOT has been recognized as a potential adjunctive therapy for the treatment of diabetic foot ulcers.
A random-effects meta-analysis of four RCTs showed that TOT improved wound healing at 12 weeks over SOC alone (RR: 1.59; 95% CI: 1.07–2.37; p = 0.021).
Investigation of statistical heterogeneity indicated that older TOT technology might be the reason for earlier poor results.
The overall GRADE level of evidence for TOT was moderate.
Supplementary Material
Abbreviations and Acronyms
- AE
adverse event
- CDO
continuous diffusion of oxygen
- CI
confidence interval
- CTO
continuous topical oxygen
- DFU
diabetes-related foot ulcer
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- J2R
jump-to-reference
- PRISMA-P
Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols
- RCT
randomized controlled trial
- RR
risk ratio
- SAE
serious adverse event
- SOC
standard of care
- TOT
topical oxygen therapy
- UT
University of Texas
AUTHORs' CONTRIBUTIONS
Marissa Carter: Data curation (lead), formal analysis (lead), investigation (supporting), methodology (lead), supervision (equal), visualization (supporting), writing—original draft (supporting), and writing–review and editing (equal). Robert Frykberg: Data curation (supporting), formal analysis (supporting), investigation (lead), methodology (supporting), writing—original draft (supporting), and writing—review and editing (equal). Alisha Oropallo: Data curation (supporting), formal analysis (supporting), investigation (supporting), methodology (supporting), writing—original draft (supporting), and writing—review and editing (equal). Chandan Sen: Investigation (supporting), methodology (supporting), and writing—review and editing (equal).
David Armstrong: Data curation (supporting), investigation (supporting), and writing—review and editing (equal). Harikrishna Nair: Writing—original draft (supporting) and writing—review and editing (equal). Thomas Serena: Conceptualization (lead), funding acquisition (lead), supervision (equal), visualization (supporting), writing—original draft (supporting), and writing—review and editing (equal). Marissa Carter takes full responsibility for the work, the study design, had access to data, and made the decision to submit and publish the article.
ACKNOWLEDGMENTS AND FUNDING SOURCES
The authors acknowledge the valuable input of Prof. Michael Edmonds (Kings College Hospital, London, United Kingdom) and the editorial assistance of Kristen Eckert, Strategic Solutions, Inc. The study was funded by SerenaGroup Research Foundation (Cambridge, MA); the study sponsor's role was to organize the study and maintain flow of work, not to influence how decisions were taken.
AUTHOR DISCLOSURE AND GHOSTWRITING
M.J.C.: Has received an honorarium from EO2 Concepts. R.G.F.: Has received research support and consulting fees from Advanced Oxygen Therapy, Inc. A.O.: Has received research support from EO2 Concepts. C.K.S.: No conflicts of interest. D.G.A.: No conflicts of interest. H.K.R.N.: No conflicts of interest. T.E.S.: Has received research support from Inotec and EO2 Concepts. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
ABOUT THE AUTHORS
Marissa J. Carter, PhD, is president of Strategic Solutions and is a clinical trial designer and biostatistician with considerable experience in wound care. Robert G. Frykberg, DPM, recently retired from his longstanding position as Chief of Podiatry at the Phoenix VA Medical Center but continues to hold the academic rank of Professor, University of Arizona College of Medicine-Phoenix. Alisha Oropallo, MD, is a vascular surgeon and wound care physician, and the medical director of the Comprehensive Wound Healing Center and Hyperbarics at Northwell Health. Chandan K. Sen, PhD, is a J. Stanley Battersby Chair and Professor of Surgery, Director of the Indiana Center for Regenerative Medicine and Engineering (ICRME), and Editor-in-Chief of Advances in Wound Care.
David G. Armstrong, DPM, is an internationally recognized leader in the field of podiatric surgery, diabetic foot, limb preservation, tissue repair, and wound healing. Harikrishna K.R. Nair, MD, is well-known expert in the field of wound healing and is head of the Wound Care Unit, Department of Internal Medicine, Kuala Lumpur Hospital, Malaysia. Thomas E. Serena, MD, is founder and Medical Director of The SerenaGroup, a family of wound, hyperbaric and research companies, and a recognized leader in medical wound research and clinical trials.
SUPPLEMENTARY MATERIAL
REFERENCES
- 1. Pecoraro RE, Reiber GE, Burgess EM. Pathways to diabetic limb amputation: basis for prevention. Diabetes Care 1990;13:513–521. [DOI] [PubMed] [Google Scholar]
- 2. Armstrong DG, Boulton AJM, Bus SA. Diabetic foot ulcers and their recurrence. N Engl J Med 2017;376:2367–2375. [DOI] [PubMed] [Google Scholar]
- 3. Zhang P, Lu J, Jing Y, et al. . Global epidemiology of diabetic foot ulceration: a systematic review and meta-analysis. Ann Med 2017;49:106–116. [DOI] [PubMed] [Google Scholar]
- 4. Zhang Y, Lazzarini PA, McPhail SM, et al. . Global disability burdens of diabetes-related lower-extremity complications in 1990 and 2016. Diabetes Care 2020;43:964–974. [DOI] [PubMed] [Google Scholar]
- 5. Rice JB, Desai U, Cummings AK, et al. . Burden of diabetic foot ulcers for medicare and private insurers. Diabetes Care 2014;37:651–658. [DOI] [PubMed] [Google Scholar]
- 6. Schaper NC, van Netten JJ, Apelqvist J, et al. . Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev 2020;36(Suppl 1):e3266. [DOI] [PubMed] [Google Scholar]
- 7. Hingorani A, LaMuraglia GM, Henke P, et al. . The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg 2016;63(2 Suppl):3S–21S. [DOI] [PubMed] [Google Scholar]
- 8. American Diabetes Association. 11. Microvascular complications and foot care: standards of medical care in diabetes–2021. Diabetes Care 2021;44(Suppl 1):S151–S167. [DOI] [PubMed] [Google Scholar]
- 9. Frykberg RG, Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care (New Rochelle) 2015;4:560–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Frykberg RG. Topical wound oxygen therapy in the treatment of chronic diabetic foot ulcers. Medicina (Kaunas) 2021;57:917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Driver VR, Reyzelman A, Kawalec J, et al. . A prospective, randomized, blinded, controlled trial comparing transdermal continuous oxygen delivery to moist wound therapy for the treatment of diabetic foot ulcers. Ostomy Wound Manage 2017;63:12–28. [PubMed] [Google Scholar]
- 12. Niederauer MQ, Michalek JE, Liu Q, et al. . Continuous diffusion of oxygen improves diabetic foot ulcer healing when compared with a placebo control: a randomised, double-blind, multicentre study. J Wound Care 2018;27(Suppl 9):S30–S45. [DOI] [PubMed] [Google Scholar]
- 13. Serena TE, Bullock MN, Cole W, et al. . Topical oxygen therapy in the treatment of diabetic foot ulcers: a multicentre, open, randomised controlled clinical trial. J Wound Care 2021;30(Suppl 5):S1–S8. [DOI] [PubMed] [Google Scholar]
- 14. Yu J, Lu S, McLaren AM, et al. . Topical oxygen therapy results in complete wound healing in diabetic foot ulcers. Wound Repair Regen 2016;24:1066–1072. [DOI] [PubMed] [Google Scholar]
- 15. Frykberg RG, Franks PJ, Edmonds M, et al. . A multinational, multicenter, randomized, double-blinded, placebo-controlled trial to evaluate the efficacy of cyclical topical wound oxygen (TWO2) therapy in the treatment of chronic diabetic foot ulcers: the TWO2 Study. Diabetes Care 2020;43:616–624. [DOI] [PubMed] [Google Scholar]
- 16. Gottrup F, Dissemond J, Baines C, et al. . Use of oxygen therapies in wound healing. J Wound Care 2017;26(Suppl 5):S1–S43. [DOI] [PubMed] [Google Scholar]
- 17. Leslie CA, Sapico FL, Ginunas VJ, et al. . Randomized controlled trial of topical hyperbaric oxygen for treatment of diabetic foot ulcers. Diabetes Care 1988;11:111–115. [DOI] [PubMed] [Google Scholar]
- 18. Blackman E, Moore C, Hyatt J, et al. . Topical wound oxygen therapy in the treatment of severe diabetic foot ulcers: a prospective controlled study. Ostomy Wound Manage 2010;56:24–31. [PubMed] [Google Scholar]
- 19. Fries RB, Wallace WA, Roy S, et al. . Dermal excisional wound healing in pigs following treatment with topically applied pure oxygen. Mutat Res 2005;579:172–181. [DOI] [PubMed] [Google Scholar]
- 20. Gordillo GM, Roy S, Khanna S, et al. . Topical oxygen therapy induces vascular endothelial growth factor expression and improves closure of clinically presented chronic wounds. Clin Exp Pharmacol Physiol 2008;35:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kalliainen LK, Gordillo GM, Schlanger R, et al. . Topical oxygen as an adjunct to wound healing: a clinical case series. Pathophysiology 2003;9:81–87. [DOI] [PubMed] [Google Scholar]
- 22. Gordillo GM, Sen CK. Evidence-based recommendations for the use of topical oxygen therapy in the treatment of lower extremity wounds. Int J Low Extrem Wounds 2009;8:105–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hirsh F, Berlin SJ, Holtz A. Transdermal oxygen delivery to diabetic wounds: a report of 6 cases. Adv Skin Wound Care 2009;22:20–24. [DOI] [PubMed] [Google Scholar]
- 24. Sen CK, Khanna S, Gordillo G, et al. . Oxygen, oxidants, and antioxidants in wound healing: an emerging paradigm. Ann N Y Acad Sci 2002;957:239–249. [DOI] [PubMed] [Google Scholar]
- 25. Nataraj M, Maiya AG, Karkada G, et al. . Application of topical oxygen therapy in healing dynamics of diabetic foot ulcers—a systematic review. Rev Diabet Stud 2019;15:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thanigaimani S, Singh T, Golledge J. Topical oxygen therapy for diabetes-related foot ulcers: a systematic review and meta-analysis. Diabet Med 2021;38:e14585. [DOI] [PubMed] [Google Scholar]
- 27. Connaghan F, Avsar P, Patton D, et al. . Impact of topical oxygen therapy on diabetic foot ulcer healing rates: a systematic review. J Wound Care 2021;30:823–829. [DOI] [PubMed] [Google Scholar]
- 28. Moher D, Shamseer L, Clarke M, et al. . Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sterne JAC, Savović J, Page MJ, et al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 30. Cro S, Morris TP, Kenward MG, et al. . Sensitivity analysis for clinical trials with missing continuous outcome data using controlled multiple imputation: a practical guide. Stat Med 2020;39:2815–2842. [DOI] [PubMed] [Google Scholar]
- 31. Siemieniuk R, Guyatt G. What is GRADE? BMJ Best Practice. https://bestpractice.bmj.com/info/us/toolkit/learn-ebm/what-is-grade/ (last accessed February 2, 2022).
- 32. Neyerlin KC, Gasteiger HA, Mittelsteadt CK, et al. . Effect of relative humidity on oxygen reduction kinetics in a PEMFC. J Electrochem Soc 2005;152:A1073. [Google Scholar]
- 33. Driver VR, Couch KS, Eckert KA, et al. . The impact of the SARS-CoV-2 pandemic on the management of chronic limb-threatening ischemia and wound care. Wound Repair Regen 2022;30:7–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaufman H, Gurevich M, Tamir E, et al. . Topical oxygen therapy used to improve wound healing in a large retrospective study of wounds of mixed aetiology. Wounds Int 2021;12:63–68. [Google Scholar]
- 35. Yellin JI, Gaebler JA, Zhou FF, et al. . Reduced hospitalizations and amputations in patients with diabetic foot ulcers treated with cyclical pressurized topical wound oxygen therapy: real-world outcomes. Adv Wound Care (New Rochelle) 2021. [Epub ahead of print]; DOI: 10.1089/wound.2021.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. U.S. Food and Drug Administration. Guidance for Industry: Chronic Cutaneous Ulcer and Burn Wounds–Developing Products for Treatment, 2006. www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm071324.pdf (last accessed February 2, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.