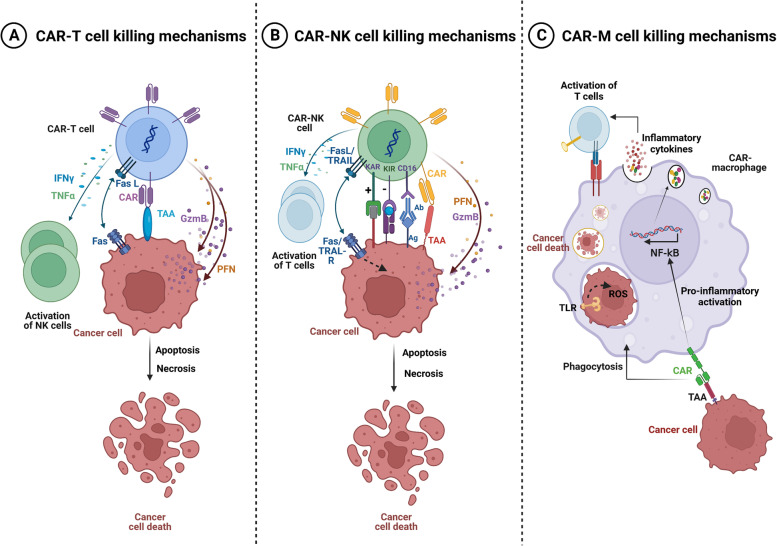
Fig. 1.
Killing mechanisms of CAR-T, CAR-NK, and CAR-M cells. A Tumor killing mechanisms of CAR-T cells. Activated CAR-T cells can specifically recognize the tumor associated antigen (TAA). Cytotoxic activity of Chimeric Antigen Receptor (CAR)-T cells is mediated by perforin (PFN) and granzyme (GzmB) granules secretion, and by activation of death receptor pathways such as Fas/Fas-L leading to cancer cells apoptosis and necrosis. Activated CAR-T cells also secrete Interferon-gamma (IFN-γ) and tumor necrosis factor-alpha (TNFα) which can promote Natural Killer (NK) cell anti-tumor cytotoxic activity. B Tumor killing mechanisms of CAR-NK cells. The activity of CAR-NK cells is regulated by the signal of activating (KAR) and inhibitory receptors (KIR) expressed on NK cells. Activated CAR-NK cells secrete the cytotoxic proteins perforin and granzyme B which synergize to induce cancer cell necrosis and apoptosis. NK cells also express the death ligands FasL and TRAIL which will bind to Fas and TRAIL-R on cancer cells and induce apoptosis. Moreover, CAR-NK cells trigger ADCC through the CD16 Fc receptor which recognize antibody-opsonized cancer cells. In addition, CAR-NK cells secrete IFN-γ and TNFα which promote their activation and stimulate other T-lymphocytes leading to increased anti-tumor immune response. NK: cell-Natural killer cells; IFN-γ: Interferon-gamma; TNFα: Tumor necrosis factor-alpha; TRAIL-R: TNF-related apoptosis-inducing ligand, KIR: Killer Inhibitory Receptors, KAR: Killer Activation Receptor, ADCC: Antibody-dependent cellular cytotoxicity, (PFN) perforin and (GzmB) granzyme. C Tumor killing mechanisms of CAR-M. The binding of a specific tumor associated antigen (TAA) with CAR receptor on the surface of CAR-M generates activation signals that mediate tumor phagocytosis, activation of transcription factors such as NF-kB and subsequent release of pro-inflammatory cytokines, which in turn can activate T cell-mediated immunity against the tumor