Abstract
Persistent cognitive and mood impairments in Gulf War Illness (GWI) are associated with chronic neuroinflammation, typified by hypertrophied astrocytes, activated microglia, and increased proinflammatory mediators in the brain. Using a rat model, we investigated whether a simple lifestyle change such as moderate voluntary physical exercise would improve cognitive and mood function in GWI. Because veterans with GWI exhibit fatigue and post-exertional malaise, we employed an intermittent voluntary running exercise (RE) regimen, which prevented exercise-induced stress. The GWI rats were provided access to running wheels three days per week for 13 weeks, commencing ten weeks after the exposure to GWI-related chemicals and stress (GWI-RE group). Groups of age-matched sedentary GWI rats (GWI-SED group) and naïve rats were maintained parallelly. Interrogation of rats with behavioral tests after the 13-week RE regimen revealed improved hippocampus-dependent object location memory and pattern separation function and reduced anxiety-like behavior in the GWI-RE group compared to the GWI-SED group. Moreover, 13 weeks of RE in GWI rats significantly reversed activated microglia with short and less ramified processes into non-inflammatory/antiinflammatory microglia with highly ramified processes and reduced the hypertrophy of astrocytes. Moreover, the production of new neurons in the hippocampus was enhanced when examined eight weeks after the commencement of RE. Notably, increased neurogenesis continued even after the cessation of RE. Collectively, the results suggest that even a moderate, intermittent physical exercise has the promise to improve brain function in veterans with GWI in association with suppression of neuroinflammation and enhancement of hippocampal neurogenesis.
Keywords: Anxiety, Astrocyte hypertrophy, Cognitive and mood function, Hippocampal neurogenesis, Microglial activation, Neuroinflammation, Object location memory, Pattern separation, Running exercise
1. Introduction
Even after three decades since the first Gulf War, over a third of 700,000 United States service personnel who served in that war are still suffering from Gulf War Illness (GWI). GWI is a multisymptom illness linked mainly to the central nervous, musculoskeletal, and gastrointestinal systems (Golomb, 2008, White et al., 2016; Coughlin and Sullivan, 2018; Dickey et al., 2020). The central nervous system (CNS) related impairments include reduced cognitive function, memory problems, mood and sleep disturbances, chronic pain, and fatigue (Odegard et al., 2013; Rayhan et al., 2013; Hubbard et al., 2014; Janulewicz et al., 2017). Epidemiological studies have suggested that intake of the nerve gas prophylactic drug pyridostigmine bromide (PB), heavy exposure to one or more chemicals during the war, or the interaction of chemical exposures with the war-related stress, underlie GWI (Steele et al., 2012; White et al., 2016; Bjørklund et al., 2020). The veterans were exposed to multiple chemicals, including the mosquito-repellant N, N-diethyl-m-toluamide (DEET), insecticide permethrin (PER), several pesticides, fuels from burning oil wells, depleted uranium, and low-doses of sarin released in the atmosphere during the combat (Golomb, 2008; White et al., 2016; Dickey et al., 2020).
Several animal model studies, using 10–28 days exposure to one or more GWI-related chemicals or a combination of a few chemicals with or without stress, have demonstrated the significant chronic symptoms of GWI (Abdel-Rahman et al., 2002; Abdullah et al., 2011; Parihar et al., 2013; O’Callaghan et al., 2015; Zakirova et al., 2015; Phillips and Deshpande, 2016; Locker et al., 2017; Miller et al., 2018). Our laboratory, using a rat model involving 28-day exposure to low-doses of PB (oral), DEET (dermal), and PER (dermal) with mild to moderate stress, has recapitulated the major symptoms of GWI with chronic cellular and molecular alterations in the brain. The most salient features of this prototype include persistent cognitive and mood dysfunction associated with several chronic changes in the hippocampus, which include decreased neurogenesis, incessantly elevated oxidative stress, and chronic inflammation typified by astrocyte hypertrophy, activated microglia, and increased concentration of proinflammatory mediators (Hattiangady et al., 2014; Shetty et al., 2017, 2020; Kodali et al., 2018; Madhu et al., 2019). Because reduced neurogenesis, elevated oxidative stress, and chronic inflammation in the hippocampus could contribute to persistent cognitive and mood impairments (Kohman and Rhodes, 2013; Peng and Bonaguidi, 2018), our previous preclinical studies in the above rat model of GWI have investigated the efficacy of antioxidant and/or antiinflammatory drugs and suggested that such therapies have the promise to improve brain function in veterans afflicted with GWI (Kodali et al., 2018; Shetty et al., 2020; Madhu et al., 2021).
In this study, we examined whether a simple lifestyle change such as moderate, intermittent voluntary physical exercise (PE) would improve cognitive and mood function in the rat model of GWI. We chose voluntary running exercise (RE) because of its ability to improve cognitive function, diminish the adverse glial changes and enhance hippocampal neurogenesis in a variety of animal models (Van Praag et al., 1999a; van Praag et al., 1999b; Kannangara et al., 2011; Hamilton and Rhodes, 2015; Ahn et al., 2017; Kodali et al., 2016; Ryan and Kelly, 2016). Since veterans with GWI display fatigue and post-exertional malaise (Lindheimer et al., 2020), we employed an intermittent voluntary RE regimen, in which the GWI rats were provided access to running wheels three days per week for 13 weeks. Such an intermittent, voluntary PE regimen employed in the study would likely avoid stress to GWI rats compared to the forced RE regimen involving treadmill with or without shocks (Svensson et al., 2016). Multiple previous studies have also shown beneficial effects of voluntary RE, including chronic stress models (Greenwood et al., 2012; Lee et al., 2016). Interrogation of GWI rats with behavioral tests after the 13-week RE regimen in this study demonstrated improved hippocampus-dependent cognitive function and reduced anxiety-like behavior associated with reversion of activated microglia with short processes into non-inflammatory/antiinflammatory microglia with highly ramified processes, reduced astrocyte hypertrophy, and enhanced neurogenesis.
2. Materials and methods
2.1. Animals
Eight-week-old male Sprague Dawley rats (n = 24) bought from Harlan (Indianapolis, IN) were kept in the vivarium, with ad libitum access to rat chow and water. Animals were next randomly ascribed to either the naïve control group (n = 8) or the GWI group (n = 16). A combined institutional animal care and use committee of the Texas A&M College of Medicine and Olin E. Teague Veterans’ Medical Center authorized all experiments performed in this study.
2.2. Study design
The various experiments and timelines are illustrated (Fig. 1 [A]). The details comprise daily exposure to GWI-related chemicals (GWIR-Cs) and moderate stress for 4 weeks, voluntary intermittent RE for 13 weeks (3 days/week) commencing 10 weeks post-exposure. The animals received daily 5′-bromodeoxyuridine (BrdU) injections for 7 days in the 8th week of the RE regimen (a time-point equivalent to 18 weeks post-exposure). Immediately after the RE regimen, the animals were interrogated with behavioral tests for assessing cognitive and mood function (i.e., at 22–28 weeks post-exposure) and brain tissue analysis for neurogenesis, astrocytes, and microglia at 28 weeks post-exposure.
Fig. 1. An overview of the experimental design and the extent of running exercise (RE).
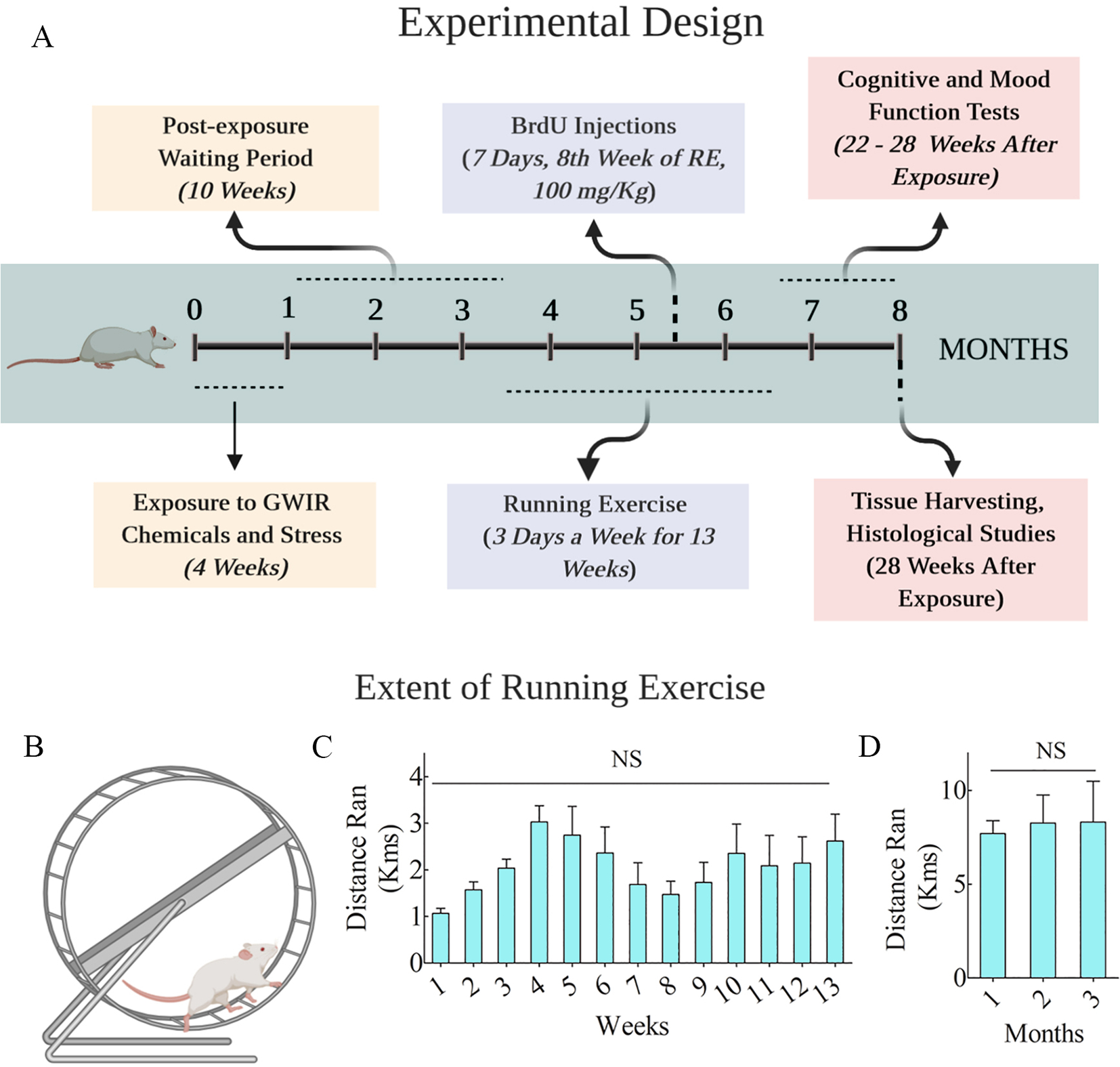
Cartoon A shows the timeline of various experiments, including rats’ exposure to Gulf War Illness-related chemicals (GWIR-Cs) and stress, RE regimen, and duration, cognitive and mood function tests, and brain tissue analyses. Animals had access to running wheels to perform voluntary RE for 13 weeks (3 times/week), commencing 10 weeks after the exposure to GWIR-Cs and stress. The behavioral tests were performed after the RE regimen, after which the brain tissues were collected for histological studies. The lower half of the figure illustrates a cartoon of the running wheel (B), the distances ran by GWI rats per week over 13 weeks (C) and per month over three months (C). NS, not significant.
2.3. Exposure of animals to GWIR-Cs and stress
The chemicals DEET and PER were purchased from Chemical Service Incorporated (West Chester, PA), whereas the drug PB was purchased from Sigma (St. Louis, MO). Animals chosen for the GWI group (n = 16) were exposed daily to DEET, PER, and PB and 15 min of restraint stress for 4 weeks (Abdel-Rahman et al., 2002, Parihar et al., 2013, Hattiangady et al., 2014, Shetty et al., 2017). The chemicals DEET (200 μl, 60 mg/Kg) and PER (200 μl, 0.2 mg/Kg) were applied dermally over shaved skin areas located on the dorsal surface of the neck and the upper thoracic region between scapulae. The drug PB was administered via oral gavage (500 μl, 2 mg/Kg). Age-matched naïve control animals (n = 8), maintained parallel to GWI animals, did not receive exposure to chemicals or stress.
2.4. Voluntary running exercise paradigm
Ten weeks after exposure to GWIR-Cs and stress, GWI-rats were randomly assigned to either the RE group (GWI-RE group, n = 9) or the sedentary group (GWI-SED group, n = 7).
Each animal in the GWI-RE group was provided intermittent access to running wheels (three days per week, Monday, Wednesday, and Friday) for 13 weeks. Animals in the GWI-RE group were housed in regular cages on non-exercise days. Animals in the GWI-SED group and naïve group were continuously housed in regular cages.
2.5. Behavioral tests for analyses of cognitive and mood function
Animals in all groups were interrogated with a series of behavioral tests to discern their cognitive and mood function. An object location test (OLT, a hippocampus-dependent cognitive test) measured animals’ ability to discern minor changes in the environment. A pattern separation test (PST), a cognitive test dependent on the dentate gyrus function and the extent of neurogenesis, evaluated animals’ proficiency to distinguish identical experiences from similar experiences. A novelty suppressed feeding test (NSFT) investigated the extent of motivation and anxiety-like behavior.
2.6. Object location test (OLT)
As detailed in our previous studies (Hattiangady et al., 2014, Shetty et al., 2020), the rat was first habituated to an empty open field apparatus for 5 min (Trial-1, T1). In trial-2 (T2), commencing 60 min after T1, the animal freely explored two similar objects placed on opposite sides of the arena for 5 min (Fig. 2 [A]). In trial-3 (T3), starting 90 min after T2, the animal was placed in the same open field with one of the objects relocated to a new locality (i.e., the object in a novel place; OINP) while the other object was maintained in its T2 location (i.e., the object in a familiar place; OIFP) (Fig. 2 [A]). The animal was allowed to explore objects for 5 min. The movement of the rat was continuously recorded via video-tracking using the Noldus Ethovision XT. Only animals that explored objects for ≥ 5 s in T4 were included for data analysis. A majority of animals met this criterion (n = 6–8 out of 7–9 animals per group). Time spent in exploring the OINP and the OIFP in T3 were acquired and compared within groups to discern the propensity of animals in each group to prefer the OINP over the OIFP. The total object exploration times (TOETs) were compared across the three groups to determine variances in object exploration between groups. Furthermore, data such as the total distances moved, and movement velocities were compared across the three groups to determine whether one or more groups displayed motor impairment.
Fig. 2. Thirteen weeks of moderate, voluntary, intermittent running exercise (RE) improved cognitive and mood function in rats with Gulf War Illness (GWI).
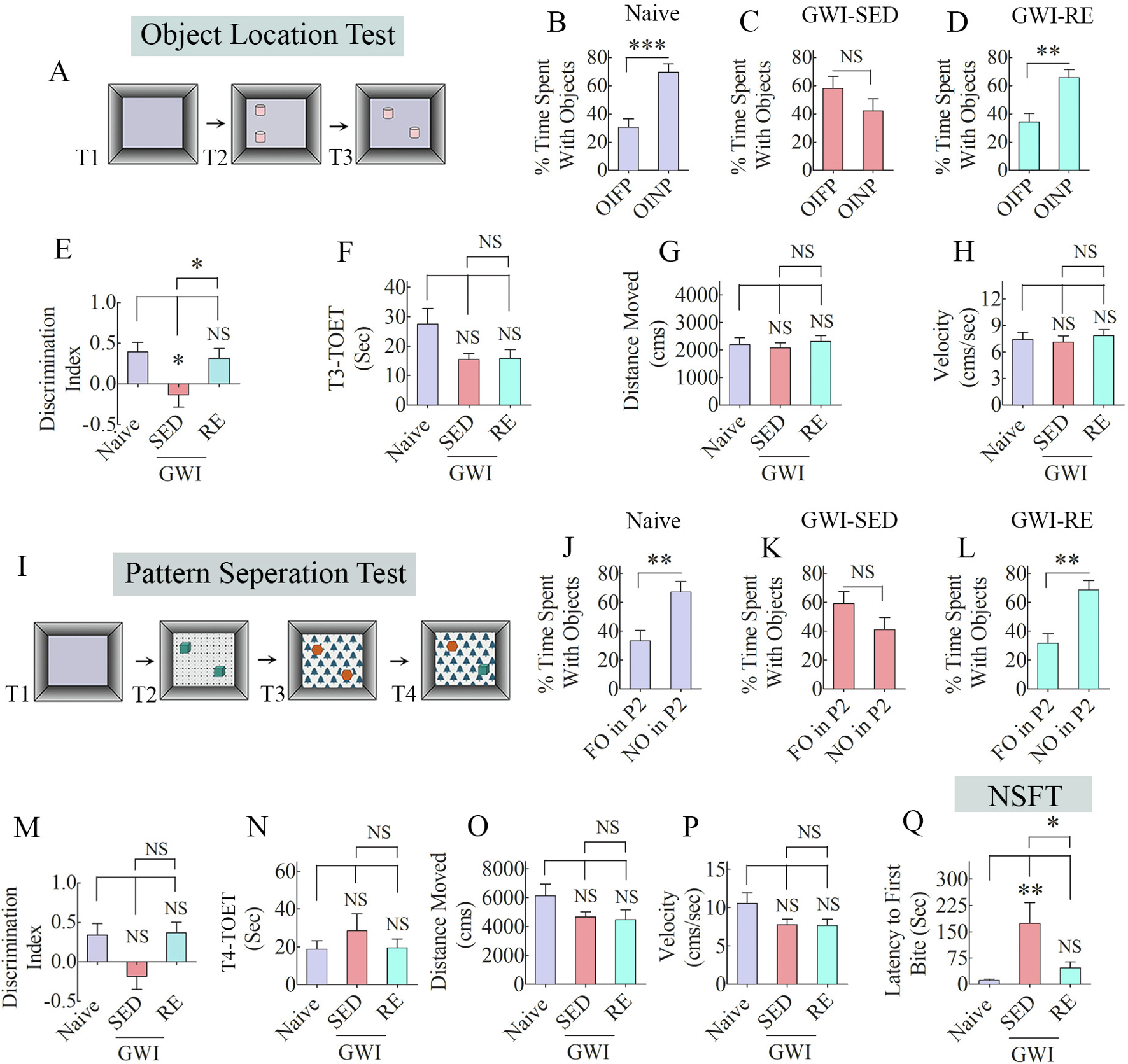
The bar charts in the top two rows show the results of an object location test (OLT). Cartoon A shows the various phases involved in OLT, whereas the bar charts in B-D show the performance of animals belonging to naïve, sedentary GWI rats (GWI-SED) or GWI rats that performed RE (GWI-RE). The bar chart E compares the discrimination index across the three groups. The bar charts F-H compare the total object exploration times (TOETs; F), the distance traveled (G), and velocity of movement (H) in trial-3 (T3) between the three groups. The bar charts in rows 3–4 show the results of a pattern separation test (PST). The cartoon in I shows the various phases involved in PST, whereas the bar charts in J-L show the performance of animals belonging to different groups. Bar chart M compares the discrimination index across groups. The bar charts N-P compare TOETs (N), the distance moved (O), and the velocity of movement (P) in T4. The bar chart Q shows the results of a novelty suppressed feeding test. *, p < 0.05, **, p < 0.01, ***, p < 0.001.
2.7. Pattern separation test (PST)
As detailed in our previous study (Shetty et al., 2020), after exploring an empty open field apparatus in T1, the animal was placed in the same arena with two identical objects placed on floor pattern-1 (i.e., type 1 objects on P1) in T2 for 5 min (Fig. 2 [I]). After an inter-trail interval of 60 min, the animal was placed again in the arena with another set of identical objects placed on floor pattern 2 (i.e., type 2 objects on P2) in T3 for 5 min (Fig. 2 [I]). In T4, commencing 180 min after T3, the animal explored the arena with one of the objects from T3 remaining in its location on P2 (i.e., the familiar object on P2; FO on P2) and the other T3-object replaced with T2-object (i.e., the novel object on P2; NO on P2). The animal explored the objects for 5 min. Only animals that explored objects for ≥ 5 s in T4 were included for data analysis. A majority of animals met this criterion (n = 6–8 out of 7–9 animals per group). Time spent in exploring the NO and the FO in T4 were acquired and compared within groups to ascertain animals’ choice in each group to prefer the NO over the FO. The TOETs were compared across groups to measure differences in object exploration between groups. Moreover, the results such as the total distances moved, and movement velocities were compared across groups to determine if one or more groups exhibited motor impairment.
2.8. Novelty suppressed feeding test (NSFT)
Each animal was first subjected to fasting for 24 h (by withdrawing food pellets from the cage), but drinking water provided ad libitum during the fasting period (n = 7–9/group). Next, a single trial lasting 5 min was conducted in an open field apparatus to measure the extent of motivation or anxiety-like behavior. A shallow plastic dish containing food pellets was placed in the center of an open field apparatus, and the rat was released from one of the corners. The animal’s movement was video tracked using the Noldus Ethovision XT program to measure the latency to the first bite of food. After every trial, the open field box was cleaned with 70% alcohol, and fresh food pellets were used for each rat to eliminate any odor-related cues. The time (latency) to the first nibble of food was used as an index of motivation or anxiety-like behavior in each rat. A higher latency value implied increased anxiety-like behavior in this test.
2.9. Tissue processing and immunohistochemical methods
Animals were deeply anesthetized through exposure to isoflurane vapor and then perfused through the heart using 4% paraformaldehyde. The detailed protocols of animal perfusion and tissue processing are available in our previous reports (Hattiangady et al., 2011; Rao et al., 2008; Kodali et al., 2018). The immunohistochemical studies comprised visualization of ionized calcium-binding adaptor molecule 1-positive (IBA-1 +) microglia and glial fibrillary acidic protein-positive (GFAP +) astrocytes in every 20th section, and 5-bromodeoxyuridine-positive (BrdU +) newly born cells and doublecortin-positive (DCX +) newly born neurons in every 15th section through the entire septo-temporal axis of the hippocampus. The primary antibodies comprised goat anti-IBA-1 (1:1000, Abcam, Cambridge, MA), rabbit anti-GFAP (1:3000, Dako, Santa Clara, CA), mouse anti-BrdU (1:200, BD, San Jose, CA), goat polyclonal anti-DCX (1:250; Santa Cruz Biotechnology, Santa Cruz, CA). The secondary antibodies employed were biotinylated anti-goat, anti-rabbit, or anti-mouse IgGs (1:250, Vector Laboratories, Burlingame, CA). The avidin–biotin complex reagent and chromogens Vector Gray and 3,3′-diaminobenzidine were purchased from Vector Labs. Finally, the sections were mounted on subbed slides, counterstained with nuclear fast red or hematoxylin, processed for coverslipping using permount, and observed under a Nikon E600 microscope.
2.10. Measurement of microglial activation and astrocyte hypertrophy
Area fractions of microglia (IBA-1 + structures) and astrocytes (GFAP + structures) were measured in the DG and hippocampal CA1 and CA3 subfields using Image J (n = 6/group). Three serial sections (every 20th) were employed in each animal for these measurements (Kodali et al., 2018). Moreover, the average area occupied by individual IBA-1 + microglial cells was measured in the CA1 subfield using image J (9 microglia randomly chosen from three serial sections in each animal). Furthermore, we measured the morphology of IBA-1 + microglia and GFAP + astrocytes in the CA1 stratum radiatum of naïve, GWI-SED, and GWI-RE groups by tracing the soma and processes using Neurolucida (Microbrightfield Inc., Williston, VT), as detailed in our previous reports (Kodali et al., 2015, 2021). In each of the three groups, 30 microglia and 30 astrocytes (5 cells/animal, 6 animals/group) were individually traced in their entirety using an oil immersion 100X lens. The measurements, such as the total process length, and the number of intersections, nodes and endings, were collected. Sholl’s concentric circle analysis was performed using the NeuroExplorer component of the Neurolucida program to determine the pattern and extent of processes at different distances from the soma in microglia and astrocytes.
2.11. Quantification of hippocampal neurogenesis
All cell counts were accomplished via the optical fractionator method available in the StereoInvestigator system (Microbrightfield) interfaced with a Nikon E600 microscope through a color digital video camera (Optronics Inc., Muskogee, OK). The numbers of newly born BrdU + cells and immature DCX + neurons in the subgranular zone-granule cell layer (SGZ-GCL) were quantified (n = 6/group). The detailed procedure employed for stereological cell counting is available in our previous reports (Rao and Shetty, 2004; Hattiangady et al., 2008; Kodali et al., 2018). The extent of neuronal differentiation of newly born cells and net neurogenesis was measured through BrdU and neuron-specific nuclear antigen (NeuN) dual immunofluorescence and Z-section analysis in a Nikon confocal microscope (n = 6/group). The primary and secondary antibodies comprised mouse anti-NeuN (1:1000, EMD Millipore, Temecula, CA), rat anti-BrdU (1:250, Serotech), donkey anti-mouse IgG tagged with Alexa Flour 488 (1:200, Invitrogen, Grand Island, NY), and donkey anti-rat IgG tagged with Alexa Flour 594 (1:200, Invitrogen). Percentages of BrdU + cells that expressed NeuN were then quantified by examination of individual BrdU + cells in 1-μm thick optical Z-sections.
2.12. Statistical analysis
The animal numbers for behavioral studies per group were determined through a power analysis using G*Power software, using the effect size f as 10 (based on our previous data) and alpha as 0.05, which suggested a requirement of final data from at least 6 rats/group to obtain a power of 0.8 and above. We obtained final data from 6 to 8 rats in this study after applying the object exploration criterion. A two-tailed, unpaired, Student’s t-test in the Prism software was employed to compare two data columns. The Mann-Whitney U test was employed when standard deviations between groups were statistically significant. One-way ANOVA with Student Neuman-Keuls post hoc tests was employed for comparisons involving three or more groups. The values in the bar charts are Mean ± S.E.M., and p < 0.05 was considered statistically significant.
3. Results
3.1. The extent of RE by GWI rats
Animals in the GWI-RE group (n = 9) ran an average of 1.1–3.0 km (kms) per week and 7.7–8.3 kms per month over 13 weeks (Fig. 1 [B–D]). ANOVA analysis showed that distance ran per week or month during the 13 weeks did not change significantly (Fig. 1 [C–D]; p > 0.05). For the entire duration of 13 weeks, the rats ran an average of 26.9 ± 4 Kms.
3.2. Moderate, intermittent voluntary RE reversed object location memory dysfunction in GWI rats
The OLT provides a measure of hippocampus-dependent spatial memory and is particularly sensitive to changes in the hippocampal CA1 subfield (Barker and Warburton, 2015). The OLT relies on the animal’s innate liking for novelty without additional external reinforcement and hence efficiently detects the proficiency of animals to perceive subtle variations in their immediate environment. Animals belonging to the naive group displayed a greater propensity to explore the OINP in T3, implying an intact spatial memory function (Fig. 2 [B], t = 4.7, p < 0.001). Animals in the GWI-SED group explored both OIFP and OINP for comparable periods, suggesting location memory impairment (Fig. 2 [C], t = 1.3, p > 0.05). In contrast, animals in GWI-RE behaved similarly as naïve controls, which was evident from their exploration of the OINP for more extended periods than the OIFP (Fig. 2 [D], t = 3.7, p < 0.01). We calculated the discrimination index using the formula, DI = time spent with NO - time spent with FO/total time spent with the objects (Radiske et al., 2017; Gulinello et al., 2019) and then compared DI across groups using one-way ANOVA, which revealed significant differences between groups (Fig. 2 [D], F = 4.6, p < 0.05). Compared to the naïve control group, the DI was reduced in the GWI-SED group (p < 0.05). The DI in the GWI-RE group was comparable to the naïve group (p > 0.05) and higher than the GWI-SED group (p < 0.05). The TOET, the total distance moved, and the velocity of movement in T3 were comparable between groups (Fig. 2 [F–H], F = 0.2–0.3; p > 0.05), implying that neither motor dysfunction nor variable levels of object exploration in one or more groups influenced the test results. Thus, moderate and intermittent voluntary RE could reverse a hippocampus-dependent cognitive dysfunction in GWI rats.
3.3. Moderate, intermittent voluntary RE improved pattern separation ability in GWI rats
Pattern separation, a capability to distinguish highly similar sensory inputs into divergent representations in the hippocampus, is a natural function of the DG important for making episodic memories (Yassa and Stark, 2011). Animals in the naïve group showed a proficiency for pattern separation, which was apparent from the exploration of NO on P2 for higher durations than the FO on P2 (Fig. 2 [J], t = 3.3, p < 0.01). Animals in the GWI-SED group showed no such preference, as they spent nearly similar percentages of TOET with the NO and the FO on P2 (Fig. 2 [K], t = 1.5, p > 0.05), which suggested an impaired pattern separation function. On the other hand, animals in the GWI-RE group spent significantly higher percentages of their TOET with the NO on P2 (Fig. 2 [L], t = 3.9, p < 0.01), akin to the behavior of animals in the naive control group. Comparison of the DI across groups using one-way ANOVA revealed significant differences between groups with naïve and GWI-RE groups displaying higher values than the GWI-SED group (Fig. 2 [M], F = 3.9, p < 0.05). However, the post-hoc tests did not show statistically significant differences between groups (p > 0.05). The TOET, the total distance moved, and the velocity of movement in T4 were comparable between groups (Fig. 2 [N–P], F = 0.8–2.5, p > 0.05), implying that neither motor dysfunction nor variable levels of object exploration in one or more groups affected the test results. Thus, moderate and intermittent voluntary RE improved GWI rats’ ability to create non-overlapping representations of similar but not identical experiences in the hippocampus.
3.4. Moderate, intermittent voluntary RE improved mood function in GWI rats
The extent of anxiety-like behavior in an NSFT was assessed by measuring the latency to approach and eat a familiar food pellet in a novel environment. The test examined the animal’s competence to overcome anxiety in a new setting that engenders anxiety and the motivation to progress towards an appetitive stimulus (Merali et al., 2003). ANOVA analysis revealed significant differences between groups (Fig. 2 [Q], F = 6.6, p < 0.01). Animals in the naïve control group displayed minimal latency to take the first bite of food, compared to much longer latencies observed in animals to perform the same task in the GWI-SED group (Fig. 2 [Q], p < 0.01). Animals in the GWI-RE group quickly moved and took the first bite, a behavior closely resembling animals in the naïve control group (Fig. 2 [Q], p > 0.05). Thus, moderate and intermittent RE could restore motivation and reduce anxiety-like behavior in GWI rats.
3.5. Moderate, intermittent voluntary RE modulated microglia into non-inflammatory types displaying highly ramified processes in the hippocampus of GWI rats
The occurrence of activated microglia is another sign of chronic neuroinflammation (Kodali et al., 2018; Shetty et al., 2020). Activated microglia typically shows fewer and significantly less ramified processes and survey a reduced brain tissue area than standard resting microglia. We visualized microglia through IBA-1 staining and quantified the area fraction of IBA-1 + structures in the DG and CA1 and CA3 subfields of the hippocampus (Fig. 3 [A–V]). The morphology of microglia in the GWI-SED group in different regions (Fig. 3 [E, K, Q]) differed from the naïve group (Fig. 3 [D, J, P]) by having fewer processes and diminished ramification of processes, the features of activated microglia. However, the morphology of microglia in the GWI-RE group (Fig. 3 [F, L, R]) was comparable to the naïve group (Fig. 3 [D, J, P]) in terms of the number of processes and the extensive ramification of processes, the resting microglia features. ANOVA analysis of area fraction of IBA + structures in the DG, CA1, and CA3 subfields and the entire hippocampus revealed significant differences between groups (Fig. 3 [S–V], F = 26.6–41.1, p < 0.0001). Post-hoc analyses consistently revealed a reduced area fraction of IBA-1 + structures in the GWI-SED group compared to the naïve group for all measured regions (Fig. 3 [S–V], p < 0.001). In contrast, area fractions of IBA-1 + structures were highly comparable between the GWI-RE and naïve groups for all measured regions (Fig. 3 [S–V), p > 0.05). Also, the area fraction of IBA-1 + structures in the GWI-RE group was higher than the GWI-SED group (Fig. 3 [S–V), p < 0.001). Quantification of the area occupied by individual microglia in the hippocampal CA1 subfield (n = 6/group) also revealed significant differences between groups (Fig. 3 [W], F = 33.3, p < 0.0001). Compared to the naïve group, the area occupied by individual microglia was reduced in the GWI-SED group (p < 0.001). However, in the GWI-RE group, the area occupied by individual microglia matched their counterparts in the naïve control group (p > 0.05) but was significantly greater than microglia in the GWI-SED group (p < 0.001).
Fig. 3. Thirteen weeks of moderate voluntary, intermittent running exercise (RE) diminished activated microglia in the hippocampus of rats with Gulf War Illness (GWI).
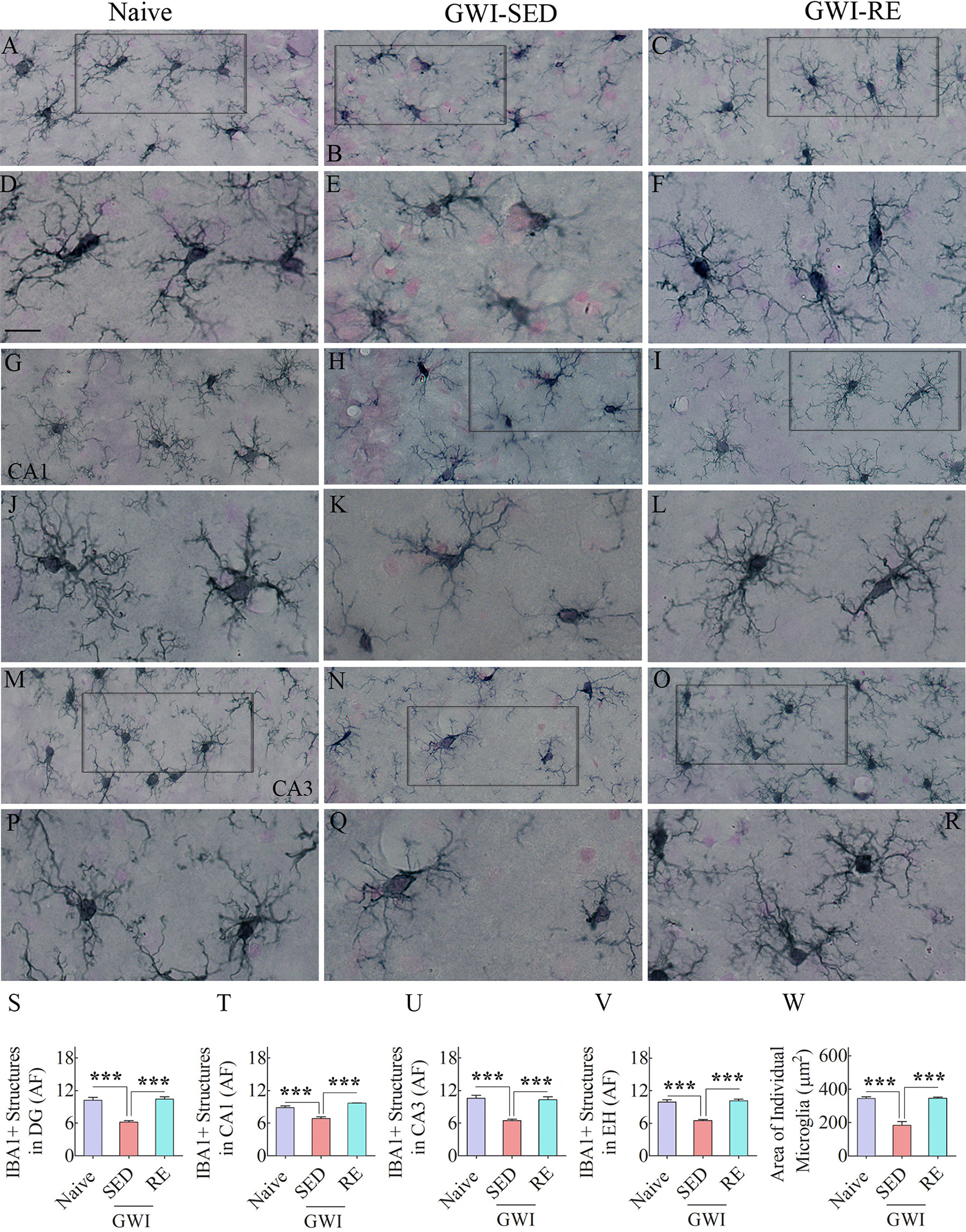
Figures A-R show examples of IBA1 + microglia from naïve control rat (A, G, M), a sedentary GWI rat (GWI-SED; B, H, N), and a GWI rat that performed RE (GWI-RE; C, I, O) in the dentate gyrus (DG; A-C), the CA1 subfield (G-I), and the CA3 subfield (M–O) of the hippocampus. D-F, J-L, and P-R are magnified views of microglia from A-C, G-I, and M–O. The bar charts S-V compare the area fraction (AF) of IBA1 + structures in the DG (S), CA1 subfield (T), CA3 subfield (U), and the entire hippocampus (EH; V) between different groups. The bar chart W compares the area occupied by individual microglia in the CA1 subfield between different groups. Scale bar, A-C, G-I, and M–O = 25 μm; D-F, J-L, and P-R = 50 μm ***, p < 0.001.
Fig. 4 illustrates examples of traced microglia from different groups (Fig. 4 [A–F]). Morphometric analyses of traced microglia suggested the presence of M1 phenotypes in the hippocampus of GWI-SED rats, compared to microglia in naïve control rats [Fig. 4 [A–B, D–E]. In contrast, GWI rats that underwent RE regimen displayed microglia resembling an M2 phenotype, akin to microglia seen in naïve control rats [Fig. 4 [A, C, D, F]. The M2 features of microglia in the naïve and GWI-RE groups include the increased area occupied by individual microglia, a higher number of nodes and endings compared to microglia in the GWI-SED group (Fig. 4 [G, I, J] F = 14.8–41.8, p < 0.001–0.00001). One-way ANOVA analysis for the total process length also revealed significant differences between groups (Fig. 4 [H] F = 3.8, p < 0.05). Post-hoc analysis showed no significant differences between the naïve and GWI-SED groups (p > 0.05) but revealed that RE enhanced the total process length of microglia in GWI rats compared to the GWI-SED group (p < 0.05). Furthermore, Sholl analysis demonstrated that microglia in the GWI-RE group exhibited a higher number of intersections (at 0–50 μm distances), nodes (0–60 μm distances), and endings (0–60 μm distances), and increased process length (at 0–50 μm distances), in comparison to the GWI-SED group (Fig. 4 [K–N], F = 9.7–38.4, p < 0.05–0.0001). Thus, 13 weeks of moderate, intermittent voluntary RE modified activated microglia into noninflammatory resting microglia in the hippocampus of GWI rats.
Fig. 4. Thirteen weeks of moderate voluntary, intermittent running exercise (RE) resulted in microglia with highly ramified processes in the hippocampus.
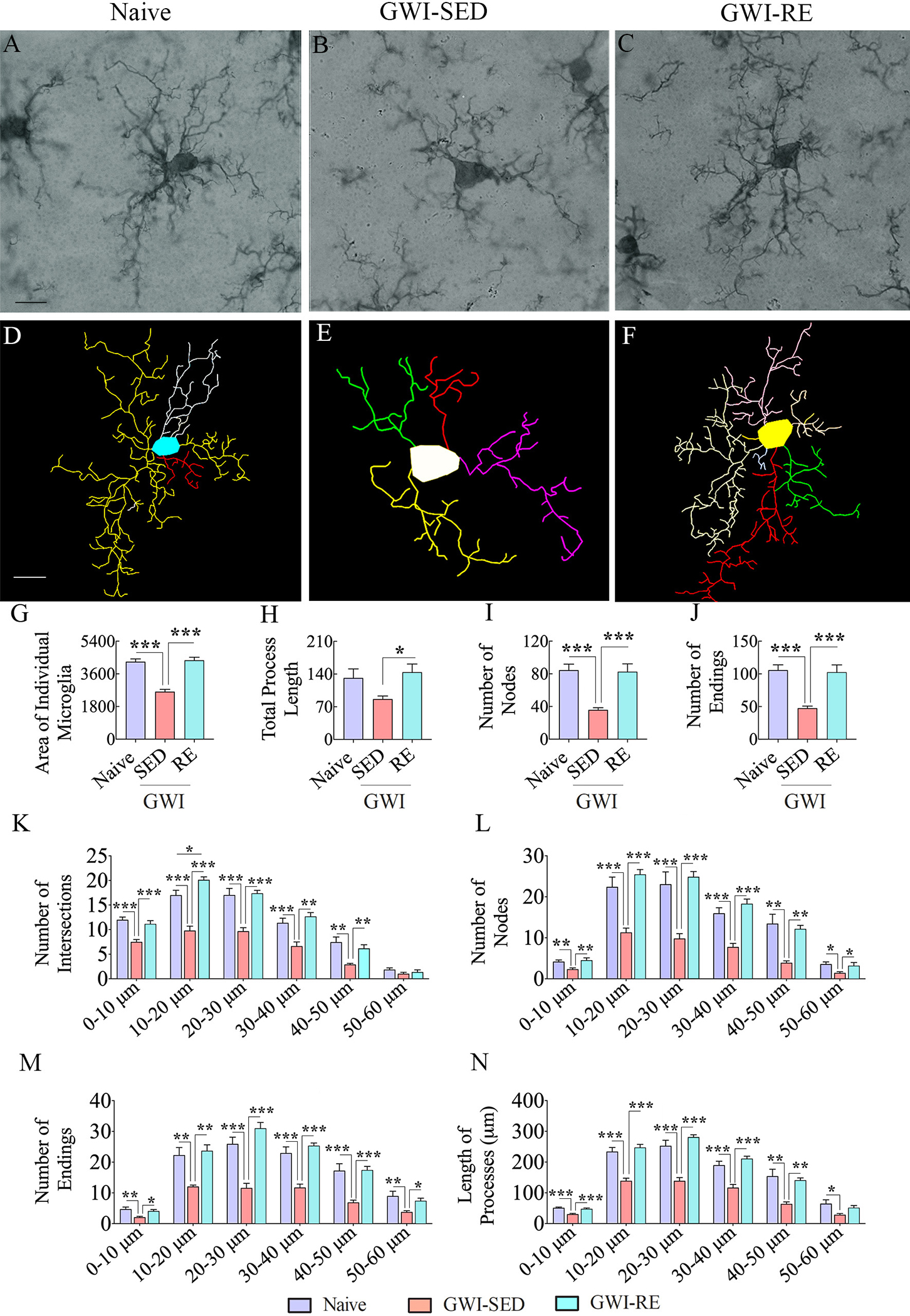
A-F shows representative examples of microglial morphology traced with Neurolucida from the hippocampus of naive (A, D), GWI-SED (B, E), and GWI-RE (C, F) groups. The bar charts G-J compare the various morphometric measures of microglia between naïve, GWI-SED, and GWI-RE groups, which include the average area occupied by individual microglia (G), the total process length (H), the number of nodes (I), and the number of process endings (J). The bar charts K-O compare the number of intersections (K), total process length (L), surface area (M), number of nodes (N), and number of process endings (O) between naïve, GWI-SED, and GWI-RE groups at 0–10 μm, 10–20 μm, 20–30 μm, 30–40 μm, 40–50 μm, and 50 – 60 μm distances from the soma. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Scale bar, A-F = 25 μm.
3.6. Moderate, intermittent voluntary RE reduced astrocyte hypertrophy in the hippocampus of GWI rats
The presence of astrocyte hypertrophy (i.e., reactive astrocytes) is one of the signs of chronic neuroinflammation in the hippocampus of GWI rats (Parihar et al., 2013; Madhu et al., 2019). We visualized astrocytes through GFAP immunostaining and quantified the area fraction of GFAP + structures in the DG, and CA1 and CA3 subfields of the hippocampus (Fig. 5 [A–L]). In the DG, the area fraction of GFAP + structures did not differ between groups (Fig. 5 [D], F = 1.1, p > 0.05). However, the area fraction of GFAP + structures in the hippocampal CA1 and CA3 subfields differed between groups (Fig. 5 [H, L], F = 4.1–6.3, p < 0.05). Post-hoc analysis revealed the increased density of hypertrophied astrocytes in the CA1 and CA3 subfields of GWI-SED compared to the naïve control group (p < 0.05–0.01). In contrast, the area fraction of GFAP + structures in the GWI-RE group was normalized to the level observed in the naïve control group (p > 0.05).
Fig. 5. Thirteen weeks of moderate, voluntary, intermittent running exercise (RE) diminished astrocyte hypertrophy in the hippocampus of rats with Gulf War Illness (GWI).
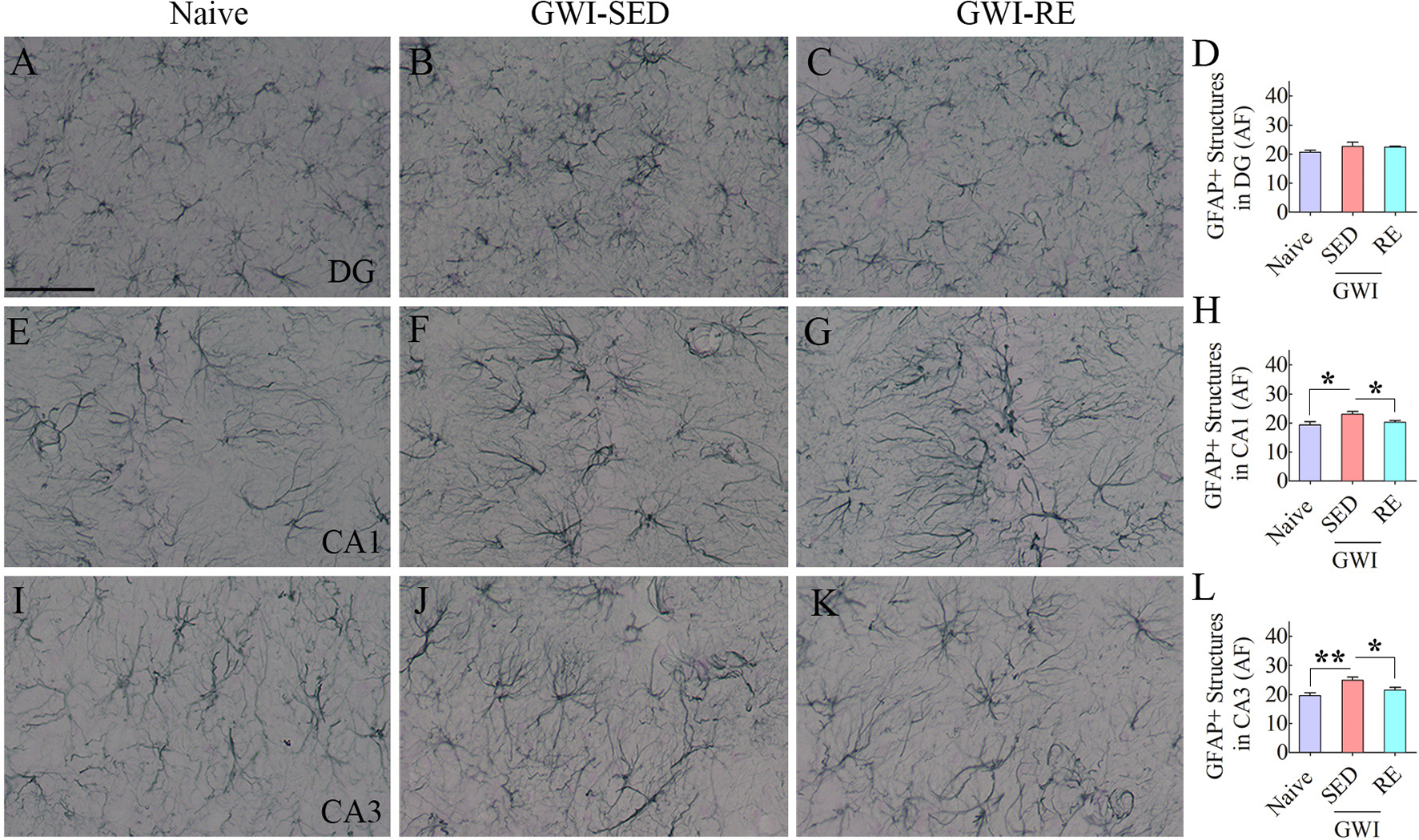
The figure shows examples of GFAP + astrocytes from a naïve control rat (A, E, I), a sedentary GWI rat (GWI-SED; B, F, J), and a GWI rat that performed RE (GWI-RE; C, D, K) in the dentate gyrus (DG; A-C), the CA1 subfield (E-G), and the CA3 subfield (I-K) of the hippocampus. The bar charts D, H, and L compare the area fraction (AF) of GFAP + structures in the DG (D), the CA1 subfield (H), and the CA3 subfield (L) of the hippocampus between different groups. Scale bar, A-C, E-G, I-K = 50 μm; *, p < 0.05, and **, p < 0.01.
Fig. 6 illustrates examples of traced astrocytes from different groups (Fig. 6 [A–F]). Morphometric analyses of traced astrocytes revealed significant differences between groups for the area of the brain tissue occupied by individual astrocytes, the total process length, and the number of nodes and endings per astrocyte (Fig. 6 [G–J], F = 6.0–12.8, p < 0.05–0.01). Compared to naïve controls, the GWI-SED group displayed increased values for all of these parameters (Fig. 6 [G–J], p < 0.05–0.001). In contrast, astrocytes in GWI rats that underwent RE displayed features comparable to astrocytes in naïve control rats (Fig. 6 [G–J], p > 0.05). All measurements in the GWI-RE group were reduced compared to the GWI-SED group (Fig. 6 [G–J], p < 0.05–0.01), implying that intermittent voluntary RE reduced the thickening and lengthening of astrocyte processes. In addition, Sholl analysis of astrocyte processes revealed that compared to the GWI-SED group, astrocytes in the GWI-RE group exhibited reduced intersections (at 20–40 μm distances), nodes (at 0–20 μm and 30–40 μm distances), and length of processes (at 0–20 μm and 30–50 μm distances (Fig. 6 [K, L, N], F = 4.4–9.3, p < 0.05–0.001). Furthermore, all Sholl measurements were comparable between the naive group and the GWI-RE group (Fig. 6 [K–N], p > 0.05). Thus, 13 weeks of intermittent RE in GWI rats reduced reactive astrocytes in the hippocampus of GWI rats.
Fig. 6. Thirteen weeks of moderate voluntary, intermittent running exercise (RE) reduced reactive astrocyte-like morphology in the hippocampus.
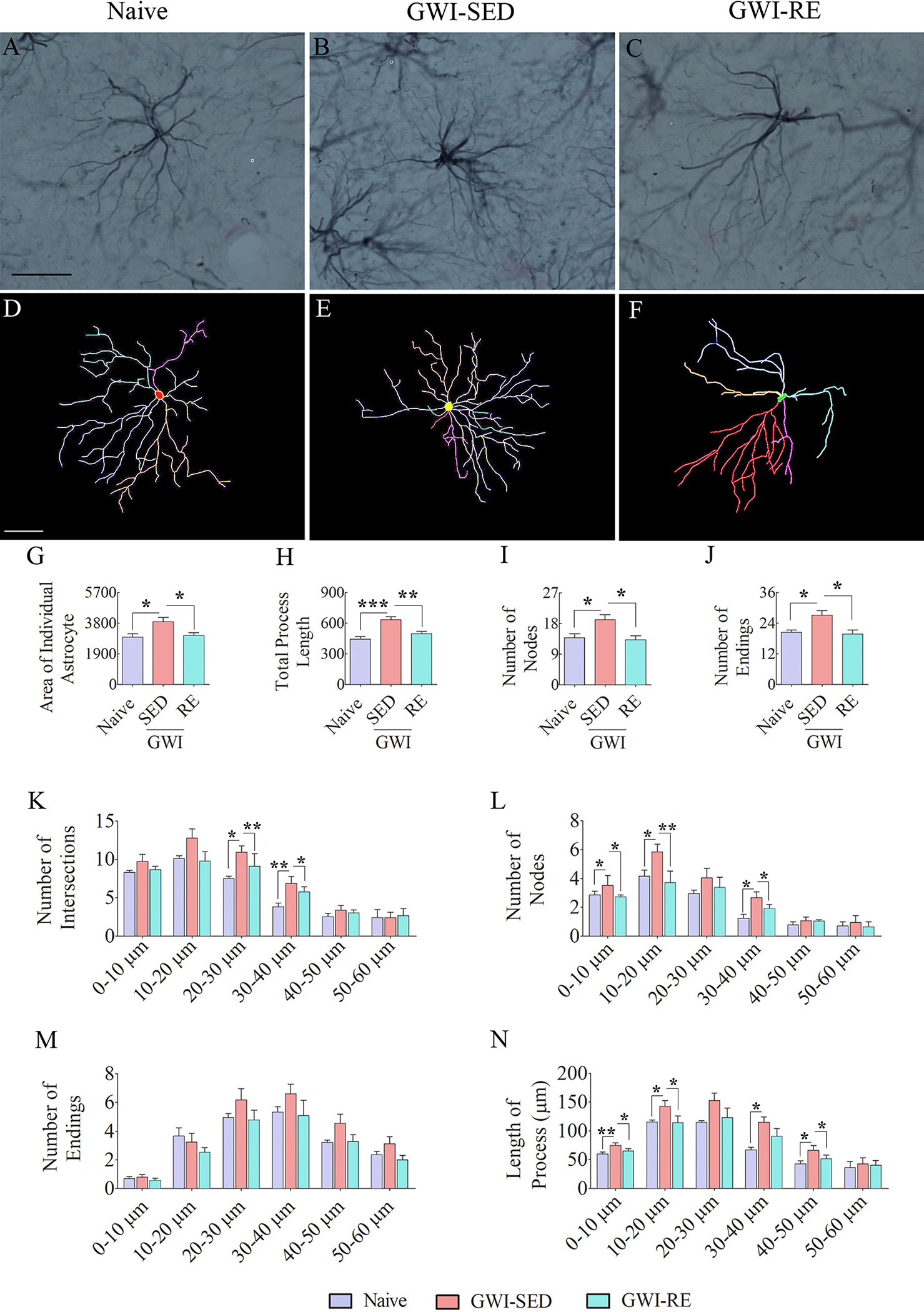
A-F shows representative examples of astrocyte morphology traced with Neurolucida from the hippocampus of naive (A, D), GWI-SED (B, E), and GWI-RE (C, F) groups. Note that astrocytes from GWI-SED rats display longer processes with enhanced branching (B, E), whereas astrocytes from naïve (A, D) and GWI-RE rats (C, F) show shorter and less branched processes. The bar charts G-J compare the various morphometric measures between naïve, GWI-SED, and GWI-RE groups, which include the average area occupied by individual astrocytes (G), the total process length (H), the number of nodes (I), and the number of process endings (J). The bar charts K-N compare the number of intersections (K), total process length (L), surface area (M), and the number of nodes (N) between naïve, GWI-SED and GWI-RE groups at 0–10 μm, 10–20 μm, 20–30 μm, 30–40 μm, 40–50 μm, and 50 – 60 μm distances from the soma. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Scale bar, A-F = 25 μm.
3.7. Moderate, intermittent voluntary RE for 8 weeks is sufficient to enhance hippocampal neurogenesis in GWI rats
We quantified the production of newly born cells in the SGZ-GCL of the hippocampus in the 8th week of the voluntary RE regimen by administering the birth-dating marker BrdU and BrdU immunohistochemistry (Fig. 7 [A–F]). Stereological quantification revealed differences in the number of BrdU + newly born cells between groups (Fig. 7 [P], F = 53.5, p < 0.0001). Compared to the naïve control group, the numbers were reduced in the GWI-SED and GWI-RE groups (Fig. 7 [P], p < 0.001). However, the total number of BrdU + cells in the GWI-RE group was significantly higher than the GWI-SED group (Fig. 7 [B–C, E–F, P], p < 0.05). Measurement of the percentage of newly born cells that differentiated into NeuN + neurons in the SGZ-GCL suggested no differences in the neuronal fate-choice decision by newly born cells between groups (Fig. 7 [Q], F = 2.6, p > 0.05). Representative images of newly born cells that differentiated into NeuN + mature neurons in the SCZ-GCL are illustrated (Fig. 7 [G–I]). Quantification of net hippocampal neurogenesis using data such as the total number of BrdU + cells and percentages of BrdU + cells expressing NeuN revealed significant differences between groups (Fig. 7 [R], F = 66.5, p < 0.0001). Compared to the naïve control group, net neurogenesis was reduced in the GWI-SED and GWI-RE groups (Fig. 7 [R], p < 0.001). However, the extent of net neurogenesis was significantly greater in the GWI-RE group than in the GWI-SED group (Fig. 7 [R], p < 0.05). Thus, 8 weeks of moderate, intermittent voluntary RE increased hippocampal neurogenesis in GWI rats.
Fig. 7. Moderate, voluntary, intermittent running exercise (RE) enhanced hippocampal neurogenesis in rats with Gulf War Illness (GWI).
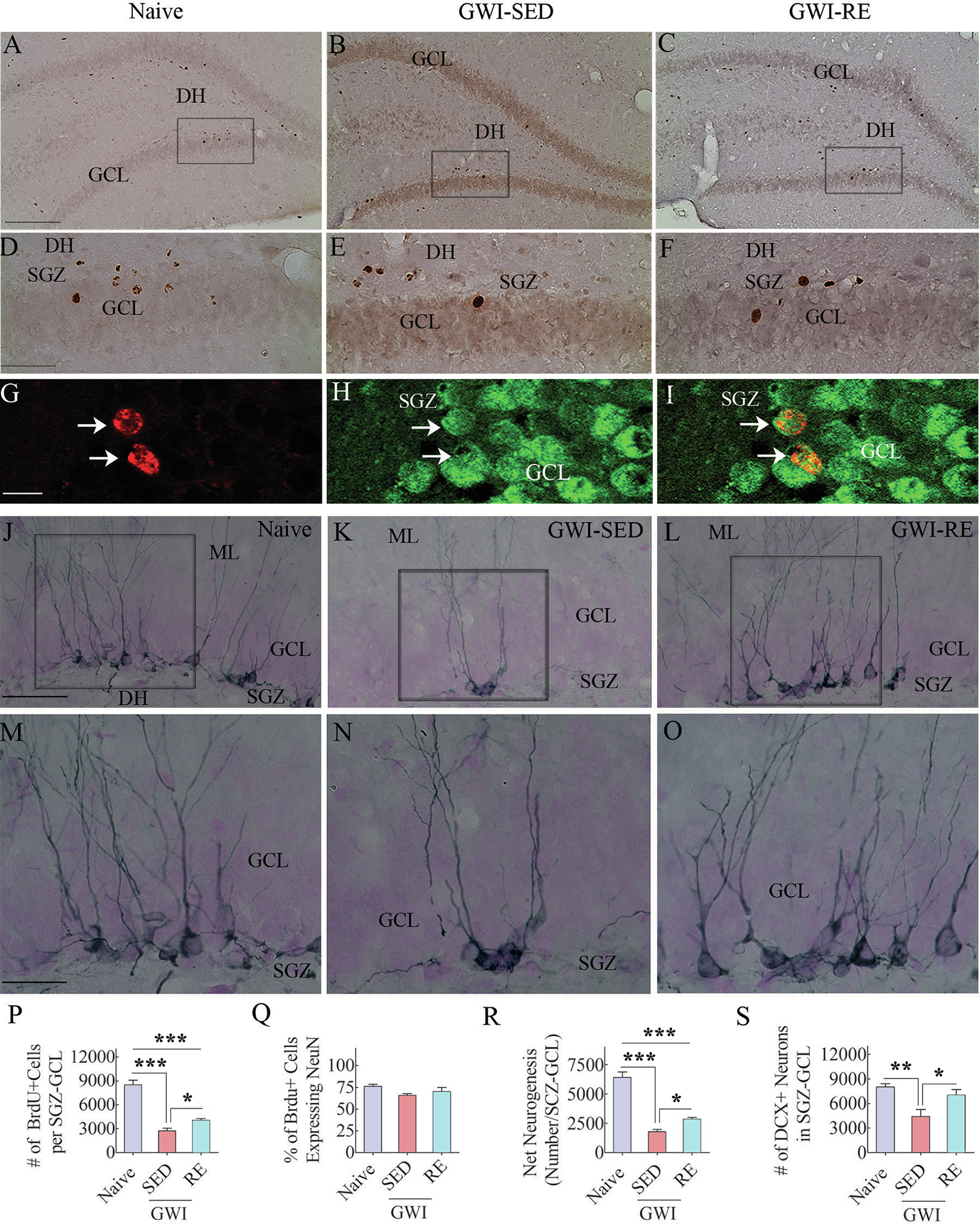
Figures in A-C show examples of 5′-bromodeoxyuridine-positive (BrdU + ) cells in the subgranular zone-granule cell layer (SGZ-GCL) of the hippocampus from a naïve control rat (A), a sedentary GWI rat (GWI-SED; B), and a GWI rats that performed RE (GWI-RE, C). D, E, and F are magnified views of regions from A, B, and C. Figures G-I illustrate examples of BrdU + cells expressing neuron-specific nuclear antigen (NeuN, arrows). The bar charts P-R compare the number of BrdU + newly born cells (P), the percentage of BrdU + cells that differentiated into NeuN + neurons (Q), and net neurogenesis (R) in the SGZ-GCL of the hippocampus between different groups. Figures J-O illustrate the distribution of doublecortin-positive (DCX + ) newly born neurons in the SGZ-GCL of the hippocampus from a naïve control rat (J), a GWI-SED rat (K), and a GWI-RE rat (L). M–O are magnified views of regions from J-L. The bar chart S compares the number of DCX + neurons across groups. *, p < 0.05; **, p < 0.01; ***, p < 0.001. GCL, granule cell layer; ML, molecular layer; DH, dentate hilus; SGZ, subgranular zone. Scale bar, A-C = 200 μm; D-F = 50 μm; G-I = 25 μm; J-L = 50 μm; M–O = 25 μm.
3.8. Moderate, intermittent voluntary RE for 13 weeks greatly enhanced hippocampal neurogenesis in GWI rats
We investigated the status of hippocampal neurogenesis in GWI rats six weeks after the cessation of the RE regimen (equivalent to 28 weeks after exposure to GWIR-Cs and stress) through DCX immunostaining of serial sections through the hippocampus (Fig. 7 [J–O]. Stereological quantification of DCX + neurons in the SGZ-GCL revealed significant differences between groups (Fig. 7 [S], F = 8.2, p < 0.01). Post-hoc tests revealed a reduced number of DCX + neurons in the GWI-SED group than the naïve group (p < 0.01). However, the number of DCX + neurons in the GWI-RE group was comparable to the naïve group (p > 0.05) and higher than the GWI-SED group (p < 0.05). Since DCX expression in newly born neurons lasts for ~14 days (Rao and Shetty 2004), DCX + cells quantified in this study represent new neurons generated 4 weeks after the termination of voluntary RE. These results imply that 13 weeks of voluntary RE is adequate to trigger a sustained enhanced activity of neural stem/progenitor cells to maintain a higher level of neurogenesis in the hippocampus of GWI rats.
4. Discussion
This study provides novel evidence that even a moderate, intermittent RE is beneficial for improving brain function in a model of GWI. Notably, improved cognitive and mood function in GWI rats was associated with modulation of inflammatory microglia displaying shorter processes lacking extensive ramifications into noninflammatory or antiinflammatory microglia with highly ramified processes, suppression of astrocyte hypertrophy, and enhanced hippocampal neurogenesis. Numerous animal model studies have shown that PE can improve cognitive function in several domains, including spatial learning (Herting and Nagel, 2012), novel object recognition memory (Hopkins et al., 2012), and cognitive flexibility (Pesce and Audiffren, 2011). Furthermore, the beneficial effects of PE on improving cognitive and/or mood function has been observed in prototypes of aging (García-Mesa et al., 2016), and neurodegenerative diseases, including Parkinson’s disease (Hsueh et al., 2018), Alzheimer’s disease (Walker et al., 2015), and Amyotrophic lateral sclerosis (Tsitkanou et al., 2019). As seen in the current study, these studies have also reported modulation of activated microglia and astrocyte hypertrophy and increased neurogenesis among the major cellular and molecular changes underlying improved brain function.
Improved cognitive and mood function following a moderate, intermittent RE observed in GWI rats has implications for improving the health of veterans afflicted with GWI as cognitive, and mood problems are among the most conspicuous CNS-related symptoms in GWI (Cooper et al., 2016; White et al., 2016; Dickey et al., 2020). There is a spectrum of cognitive problems in veterans with GWI, including visuospatial, attention, executive function, learning, and memory deficits (Janulewicz et al., 2017). Furthermore, better hippocampus-dependent function observed after RE in the current study is of interest because veterans with GWI indeed have hippocampus dysfunction, evident from studies using magnetic resonance spectroscopy and single-photon emission computed tomography (Haley et al., 2000a, 2000b, 2009; Menon et al., 2004) and arterial spin labeling with the evaluation of hippocampal function (Li et al., 2011). Memory dysfunction in GWI was also observed when a face-name associative recall test with functional MRI was performed (Odegard et al., 2013; Cooper et al., 2016). The severity of mood impairment in GWI, on the other hand, is variable but has been shown to result in mental health diagnoses in some patients (Engdahl et al., 2018). Investigations have suggested that cognitive fatigue in GWI stems from the prolonged activation of the executive control network (Wylie et al., 2019) and weakening functional connectivity (Gopinath et al., 2019). Studies in animal prototypes of GWI have also reported consistent cognitive and mood impairments and neuropathology observed in veterans with GWI (Parihar et al., 2013; Hattiangady et al., 2014; Zakirova et al., 2015; Phillips and Deshpande, 2016; Kodali et al., 2018; Shetty et al., 2020). While cognitive and mood dysfunction could occur from multiple changes in the hippocampus and other brain regions, correlative studies in veterans with GWI have suggested that altered cerebral blood flow (CBF) (Falvo et al., 2018) and neuroinflammation (Alshelh et al., 2020) likely underlie these impairments in veterans with GWI. Animal model studies have also identified increased oxidative stress and waning of hippocampal neurogenesis as other causes of cognitive and mood dysfunction in GWI (Parihar et al., 2013; Kodali et al., 2018; Shetty et al., 2017, 2020). Remarkably, PE has been shown to improve many of the above symptoms and pathology in different conditions or diseases. For instance, PE has been shown to alter CBF and improve cognitive performance in older adults with and without cognitive impairment (Alfini et al., 2019). Another study has shown that even 20 min of moderate exercise can increase CBF in the hippocampus by 10–12% (Steventon et al., 2020). While the current study did not examine CBF in GWI rats, such studies could be performed in the future on veterans with GWI after a moderate, intermittent exercise regimen.
The current study demonstrated that RE transformed putative inflammatory microglia displaying shorter processes into noninflammatory or antiinflammatory microglia with highly ramified processes in GWI rats. Proficiency of PE to reduce microglial activation has been observed earlier in several neurodegenerative disease models, including aging (He et al., 2017), Alzheimer’s disease (Nichol et al., 2008), and traumatic brain injury (Piao et al., 2013). How does PE convert activated microglia into noninflammatory or antiinflammatory microglia? Studies have shown that PE can inhibit microglia activation and neuroinflammation through multiple mechanisms (Mee-inta et al., 2019). These comprise PE-induced increases in interleukin-4 (IL-4) and IL-10, IL-1 alpha receptor (IL-1Ra), the cluster of differentiation 200 (CD200), soluble triggering receptor expressed on myeloid cells 2 (TREM2), heat shock proteins (HSPs), sirtuin-1 (SIRT1), brain-derived neurotrophic factor (BDNF), antioxidants, and glymphatic clearance. PE induces the release of antiinflammatory cytokines IL-4 and IL-10 by skeletal muscles (Bobinski et al., 2018; Calegari et al., 2018), which reach the CNS through the circulating blood (Kelly, 2018). When IL-10 acts on its receptor expressed on microglia, it enhances the suppressor of cytokine signaling 3 (SOCS3), which inhibits microglial activation (Cianciulli et al., 2015). PE increases the expression of IL-1Ra in the CNS, which leads to the binding of IL-1Ra to IL-1R expressed on microglia, which inhibits the activity of IL-1α or IL-1β and thereby reduces microglial activation (Rosenzweig et al., 2014). Furthermore, PE enhances the immunomodulatory factor CD200 in neurons, which can inhibit microglial activity through binding with CD200R (Biber et al., 2007), leading eventually to inhibition of redundant acronym syndrome /extracellular-signal-regulated kinase/mitogen-activated protein kinase (Ras/ERK-MAPK) inflammatory signaling pathways (Mee-inta et al., 2019). Moreover, PE increases the concentration of soluble TREM2, an immunoglobulin superfamily receptor expressed on microglia (Jensen et al., 2019). Activation of TREM2 leads to increased phagocytosis function in microglia and blocks MAPK cascade at the rapidly accelerated fibrosarcoma (RAF) level (Filipello et al., 2018), which inhibits inflammatory responses driven by toll-like receptor 4 (TLR4) on microglia (Mecca et al., 2018). PE can also increase the production of SIRT1, an NAD + -dependent protein deacetylase (Lai et al., 2014) capable of modulating inflammation through deacetylation of transcription factors such as nuclear factor kappa B (NF-kB) subunit-65 (Haigis and Sinclair, 2010). SIRT1 can also reduce inflammation through activation of nuclear factor erythroid 2 (NRF-2), a master regulator of antioxidant response and antagonist of NF-kB cascade (Pedruzzi et al., 2012).
PE also increases the concentration of BDNF secreted by astrocytes and microglia (Choi et al., 2018). PE can enhance BDNF in the CNS in several ways. PE-induced muscle contraction activates PGC-1α, which stimulates irisin release from its membrane-bound precursor, fibronectin type III domain-containing protein-5 (FNDC5) (Wrann et al., 2013). Then, irisin in the circulating blood enters the brain and upregulates BDNF. Apart from promoting neuronal survival, hippocampal neurogenesis, synaptic plasticity, and cognitive and mood function (Greenberg et al., 2009), increased BDNF can diminish microglial activation via inhibition of NF-κB and transcription of specific antiinflammatory genes through induction of ERK activation and cAMP-response element-binding protein (CREB) phosphorylation (Dong et al., 2018). BDNF can also modulate mitogen-activated protein kinase phosphatase 1, leading to diminished p38 and JNK phosphorylation (Jeanneteau et al., 2010). PE-induced increased BDNF is likely vital in improving brain function in GWI because BDNF is decreased in GWI models (Bose et al., 2020). PE can also modulate microglia through the upregulation of antioxidants such as glutathione peroxidase and superoxide dismutase (Radak et al., 2001). Another way PE could inhibit microglial activation is through improved glymphatic clearance (Von Holstein-Rathlou et al., 2018), a system draining the brain’s interstitial fluid into the cerebrospinal fluid and then to the cervical lymph nodes (Shetty and Zanirati, 2020). Additionally, PE can reduce microglial activation by modifying the gut microbiota because of bidirectional communication between the brain and the gut (Dinan and Cryan, 2017; Abraham et al., 2019). Since gut microbiota in GWI is negatively altered (Alhasson et al., 2017; Janulewicz et al., 2019), PE may normalize the microbiome and help improve cognitive function via suppression of microglial activation. Thus, suppression of microglial activity through multiple mechanisms is likely one of the major factors underlying RE-induced improved cognitive and mood function in GWI rats in this study. RE-induced alleviation of astrocyte hypertrophy may have also played a role in improving cognitive and mood function in GWI rats. Such conclusion is supported by studies showing an association between hypertrophied astrocytes and increased complement C3 in the brain of GWI rats (Madhu et al., 2019), and increased complement signaling mediating synaptic dysfunction and contributing to cognitive and mood dysfunction (Boyle et al., 2007; Lian et al., 2015).
Better cognitive and mood function observed in GWI rats after RE could also be due to the release of multiple other neurotrophic factors and hippocampal neurogenesis. Apart from BDNF, PE has been shown to increase insulin-like growth factor-I and vascular endothelial growth factor (Woost et al., 2018; Cotman et al., 2007), which can improve cognitive function through increased neurogenesis (Trejo et al., 2001; Fabel et al., 2003). Indeed, the current study showed that eight weeks of moderate intermittent voluntary RE increased hippocampal neurogenesis in GWI rats when examined through injections of the birth-dating marker BrdU. Additional quantification of DCX + neurons revealed that 13 weeks of voluntary RE triggered a sustained enhanced activity of neural stem/progenitor cells to maintain a higher level of hippocampal neurogenesis in GWI rats, even after the cessation of the PE regimen. While many previous studies have demonstrated the ability of PE to enhance hippocampal neurogenesis in naïve adult animals (Van Praag et al., 1999a; van Praag et al., 1999b; Brandt et al., 2010; Wolf et al., 2011), the current study provides the first demonstration of increased neurogenesis with RE in a model of GWI. Hippocampal neurogenesis occurs throughout life and has been suggested to contribute to the formation of new memories and maintaining mood and pattern separation function (Deng et al., 2010; Snyder et al., 2011; França et al., 2017). Considering the function of neurogenesis, and that diminished neurogenesis is one of the consistent changes associated with cognitive and mood dysfunction in the rat model of GWI (Parihar et al., 2013; Kodali et al., 2018; Shetty et al., 2020), it is plausible that PE-induced increased neurogenesis is another vital factor that contributed to improved cognitive and mood function in this study.
5. Conclusions and future perspectives
The study demonstrated that 13 weeks of moderate, intermittent, voluntary RE is efficacious for improving cognitive and mood function in rats with GWI. The application of moderate, intermittent, and voluntary RE regimens in this study likely avoided undue stress to GWI rats, which is an important issue because fatigue and post-exertional malaise are other features of GWI in veterans. Investigation of the hippocampus suggested that modulation of activated microglia into noninflammatory, or antiinflammatory resting microglia, alleviation of astrocyte hypertrophy, and enhanced neurogenesis likely underlie RE-induced improvements in cognition and mood. Several other molecular mechanisms are likely also involved in mediating the beneficial effects of RE, which would require additional studies in the future. In summary, the results support the application of moderate, intermittent PE to the treatment regimen in veterans with GWI, especially for those having cognitive and/or mood problems with no physical disability.
Acknowledgments
This work was mainly supported by grants from the Department of Veterans Affairs (VA Merit Award I01BX000883 to A.K.S) and Department of Defense (W81XWH-16-1-0480 and W81XWH-17-1-0447 to AKS) and partly by the National Institutes of Health Grant (R01NS106907-01 to AKS).
Department of Defense, United States Government Disclaimer
The contents of this article suggest the views of authors and do not represent the views of the Department of Defense or the United States Government.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Maheedhar Kodali: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. Vikas Mishra: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. Bharathi Hattiangady: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. Sahithi Attaluri: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. Jenny Jaimes Gonzalez: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – review & editing. Bing Shuai: Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization. Ashok K. Shetty: Conceptualization, Project administration, Funding acquisition, Supervision, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
References
- Abdel-Rahman A, Shetty AK, Abou-Donia MB, 2002. Disruption of the blood-brain barrier and neuronal cell death in cingulate cortex, dentate gyrus, thalamus, and hypothalamus in a rat model of Gulf-War syndrome. Neurobiol. Dis. 10, 306–326. [DOI] [PubMed] [Google Scholar]
- Abdullah L, Crynen G, Reed J, Bishop A, Phillips J, Ferguson S, Mouzon B, Mullan M, Mathura V, Mullan M, Ait-Ghezala G, Crawford F, 2011. Proteomic CNS profile of delayed cognitive impairment in mice exposed to Gulf War agents. Neuromol. Med. 13, 275–288. [DOI] [PubMed] [Google Scholar]
- Abraham D, Feher J, Scuderi GL, Szabo D, Dobolyi A, Cservenak M, Juhasz J, Ligeti B, Pongor S, Gomez-Cabrera MC, Vina J, Higuchi M, Suzuki K, Boldogh I, Radak Z, 2019. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 115, 122–131. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Shin MC, Park JH, Kim IH, Cho JH, Lee TK, Lee JC, Chen BH, Shin BN, Tae HJ, Park J, Choi SY, Lee YL, Kim DW, Kim YH, Won MH, Cho JH, 2017. Effects of long-term post-ischemic treadmill exercise on gliosis in the aged gerbil hippocampus induced by transient cerebral ischemia. Mol. Med. Rep. 15, 3623–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfini AJ, Weiss LR, Nielson KA, Verber MD, Smith JC, 2019. Resting Cerebral Blood Flow After Exercise Training in Mild Cognitive Impairment. J. Alzheimer’s disease : JAD 67, 671–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhasson F, Das S, Seth R, Dattaroy D, Chandrashekaran V, Ryan CN, Chan LS, Testerman T, Burch J, Hofseth LJ, Horner R, Nagarkatti M, Nagarkatti P, Lasley SM, Chatterjee S, 2017. Altered gut microbiome in a mouse model of Gulf War Illness causes neuroinflammation and intestinal injury via leaky gut and TLR4 activation. PLoS ONE 12, e0172914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alshelh Z, Albrecht DS, Bergan C, Akeju O, Clauw DJ, Conboy L, Edwards RR, Kim M, Lee YC, Protsenko E, Napadow V, Sullivan K, Loggia ML, 2020. In-vivo imaging of neuroinflammation in veterans with Gulf War illness. Brain Behav. Immun. 87, 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker GR, Warburton EC, 2015. Object-in-place associative recognition memory depends on glutamate receptor neurotransmission within two defined hippocampal-cortical circuits: a critical role for AMPA and NMDA receptors in the hippocampus, perirhinal, and prefrontal cortices. Cerebral cortex (New York, N.Y. : 1991) 25, 472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW, 2007. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 30, 596–602. [DOI] [PubMed] [Google Scholar]
- Bjørklund G, Pivina L, Dadar M, Semenova Y, Rahman MM, Chirumbolo S, Aaseth J, 2020. Depleted uranium and Gulf War Illness: Updates and comments on possible mechanisms behind the syndrome. Environ. Res. 181, 108927. [DOI] [PubMed] [Google Scholar]
- Bobinski F, Teixeira JM, Sluka KA, Santos ARS, 2018. Interleukin-4 mediates the analgesia produced by low-intensity exercise in mice with neuropathic pain. Pain 159, 437–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose D, Saha P, Mondal A, Fanelli B, Seth RK, Janulewicz P, Sullivan K, Lasley S, Horner R, Colwell RR, Shetty AK, Klimas N, Chatterjee S, 2020. Obesity Worsens Gulf War Illness Symptom Persistence Pathology by Linking Altered Gut Microbiome Species to Long-Term Gastrointestinal, Hepatic, and Neuronal Inflammation in a Mouse Model. Nutrients 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle SH, Jackson WG, Suarez EC, 2007. Hostility, anger, and depression predict increases in C3 over a 10-year period. Brain Behav. Immun. 21, 816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt MD, Maass A, Kempermann G, Storch A, 2010. Physical exercise increases Notch activity, proliferation and cell cycle exit of type-3 progenitor cells in adult hippocampal neurogenesis. Eur. J. Neurosci. 32, 1256–1264. [DOI] [PubMed] [Google Scholar]
- Calegari L, Nunes RB, Mozzaquattro BB, Rossato DD, Dal Lago P, 2018. Exercise training improves the IL-10/TNF-α cytokine balance in the gastrocnemius of rats with heart failure. Braz. J. Phys. Therapy 22, 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Bylykbashi E, Chatila ZK, Lee SW, Pulli B, Clemenson GD, Kim E, Rompala A, Oram MK, Asselin C, Aronson J, Zhang C, Miller SJ, Lesinski A, Chen JW, Kim DY, van Praag H, Spiegelman BM, Gage FH, Tanzi RE, 2018. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science (New York, N.Y.) 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianciulli A, Dragone T, Calvello R, Porro C, Trotta T, Lofrumento DD, Panaro MA, 2015. IL-10 plays a pivotal role in anti-inflammatory effects of resveratrol in activated microglia cells. Int. Immunopharmacol. 24, 369–376. [DOI] [PubMed] [Google Scholar]
- Cooper CM, Briggs RW, Farris EA, Bartlett J, Haley RW, Odegard TN, 2016. Memory and functional brain differences in a national sample of U.S. veterans with Gulf War Illness. Psych. Res. Neuroimag. 250, 33–41. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA, 2007. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 30, 464–472. [DOI] [PubMed] [Google Scholar]
- Coughlin SS, Sullivan K, 2018. Study Protocol: Southern Women Veterans’ Health Study. Annals of epidemiology and public health 1. [PMC free article] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH, 2010. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nature reviews. Neuroscience 11, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey B, Madhu LN, Shetty AK, 2020. Gulf War Illness: Mechanisms Underlying Brain Dysfunction and Promising Therapeutic Strategies. Pharmacol. Therapeut, 107716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, Cryan JF, 2017. The Microbiome-Gut-Brain Axis in Health and Disease. Gastroenterol. Clin. North Am. 46, 77–89. [DOI] [PubMed] [Google Scholar]
- Dong Y, Pu K, Duan W, Chen H, Chen L, Wang Y, 2018. Involvement of Akt/CREB signaling pathways in the protective effect of EPA against interleukin-1β-induced cytotoxicity and BDNF down-regulation in cultured rat hippocampal neurons. BMC Neurosci. 19, 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engdahl BE, James LM, Miller RD, Leuthold AC, Lewis SM, Carpenter AF, Georgopoulos AP, 2018. Brain Function in Gulf War Illness (GWI) and Associated Mental Health Comorbidities. J. Neurol. Neuromed. 3, 24–34. [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD, 2003. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur. J. Neurosci. 18, 2803–2812. [DOI] [PubMed] [Google Scholar]
- Falvo MJ, Lindheimer JB, Serrador JM, 2018. Dynamic cerebral autoregulation is impaired in Veterans with Gulf War Illness: A case-control study. PLoS ONE 13, e0205393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipello F, Morini R, Corradini I, Zerbi V, Canzi A, Michalski B, Erreni M, Markicevic M, Starvaggi-Cucuzza C, Otero K, Piccio L, Cignarella F, Perrucci F, Tamborini M, Genua M, Rajendran L, Menna E, Vetrano S, Fahnestock M, Paolicelli RC, Matteoli M, 2018. The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity 48, 979–991.e978. [DOI] [PubMed] [Google Scholar]
- França TFA, Bitencourt AM, Maximilla NR, Barros DM, Monserrat JM, 2017. Hippocampal neurogenesis and pattern separation: A meta-analysis of behavioral data. Hippocampus 27, 937–950. [DOI] [PubMed] [Google Scholar]
- García-Mesa Y, Colie S, Corpas R, Cristòfol R, Comellas F, Nebreda AR, Giménez-Llort L, Sanfeliu C, 2016. Oxidative Stress Is a Central Target for Physical Exercise Neuroprotection Against Pathological Brain Aging. J. Gerontol. Ser. A, Biol. Sci. Med. Sci. 71, 40–49. [DOI] [PubMed] [Google Scholar]
- Golomb BA, 2008. Acetylcholinesterase inhibitors and Gulf War illnesses. PNAS 105, 4295–4300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath KS, Sakoglu U, Crosson BA, Haley RW, 2019. Exploring brain mechanisms underlying Gulf War Illness with group ICA based analysis of fMRI resting state networks. Neurosci. Lett. 701, 136–141. [DOI] [PubMed] [Google Scholar]
- Greenberg ME, Xu B, Lu B, Hempstead BL, 2009. New insights in the biology of BDNF synthesis and release: implications in CNS function. J. Neurosci. Off. J. Soc. Neurosci. 29, 12764–12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Loughridge AB, Sadaoui N, Christianson JP, Fleshner M, 2012. The protective effects of voluntary exercise against the behavioral consequences of uncontrollable stress persist despite an increase in anxiety following forced cessation of exercise. Behav. Brain Res. 233, 314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Mitchell HA, Chang Q, Timothy O’Brien W, Zhou Z, Abel T, Wang L, Corbin JG, Veeraragavan S, Samaco RC, Andrews NA, Fagiolini M, Cole TB, Burbacher TM, Crawley JN, 2019. Rigor and reproducibility in rodent behavioral research. Neurobiol. Learn. Mem. 165, 106780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigis MC, Sinclair DA, 2010. Mammalian sirtuins: biological insights and disease relevance. Ann. Rev. Pathol. 5, 253–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley RW, Fleckenstein JL, Marshall WW, McDonald GG, Kramer GL, Petty F, 2000a. Effect of basal ganglia injury on central dopamine activity in Gulf War syndrome: correlation of proton magnetic resonance spectroscopy and plasma homovanillic acid levels. Arch. Neurol. 57, 1280–1285. [DOI] [PubMed] [Google Scholar]
- Haley RW, Marshall WW, McDonald GG, Daugherty MA, Petty F, Fleckenstein JL, 2000b. Brain abnormalities in Gulf War syndrome: evaluation with 1H MR spectroscopy. Radiology 215, 807–817. [DOI] [PubMed] [Google Scholar]
- Haley RW, Spence JS, Carmack PS, Gunst RF, Schucany WR, Petty F, Devous MD Sr., Bonte FJ, Trivedi MH, 2009. Abnormal brain response to cholinergic challenge in chronic encephalopathy from the 1991 Gulf War. Psychiatry Res. 171, 207–220. [DOI] [PubMed] [Google Scholar]
- Hamilton GF, Rhodes JS, 2015. Exercise Regulation of Cognitive Function and Neuroplasticity in the Healthy and Diseased Brain. Prog. Mol. Biol. Transl. Sci. 135, 381–406. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Mishra V, Kodali M, Shuai B, Rao X, Shetty AK, 2014. Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War illness. Front. Behav. Neurosci. 8, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK, 2008. Grafting of striatal precursor cells into hippocampus shortly after status epilepticus restrains chronic temporal lobe epilepsy. Exp. Neurol. 212, 468–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattiangady B, Kuruba R, Shetty AK, 2011. Acute seizures in old age leads to a greater loss of CA1 pyramidal neurons, an increased propensity for developing chronic TLE and a severe cognitive dysfunction. Aging Dis 2, 1–17. [PMC free article] [PubMed] [Google Scholar]
- He XF, Liu DX, Zhang Q, Liang FY, Dai GY, Zeng JS, Pei Z, Xu GQ, Lan Y, 2017. Voluntary Exercise Promotes Glymphatic Clearance of Amyloid Beta and Reduces the Activation of Astrocytes and Microglia in Aged Mice. Front. Mol. Neurosci. 10, 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Nagel BJ, 2012. Aerobic fitness relates to learning on a virtual Morris Water Task and hippocampal volume in adolescents. Behav. Brain Res. 233, 517–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins ME, Davis FC, Vantieghem MR, Whalen PJ, Bucci DJ, 2012. Differential effects of acute and regular physical exercise on cognition and affect. Neuroscience 215, 59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsueh SC, Chen KY, Lai JH, Wu CC, Yu YW, Luo Y, Hsieh TH, Chiang YH, 2018. Voluntary Physical Exercise Improves Subsequent Motor and Cognitive Impairments in a Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard NA, Hutchison JL, Motes MA, Shokri-Kojori E, Bennett IJ, Brigante RM, Haley RW, Rypma B, 2014. Central Executive Dysfunction and Deferred Prefrontal Processing in Veterans with Gulf War Illness. Clin. Psychol. Sci. J. Associat. Psychol. Sci. 2, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janulewicz PA, Krengel MH, Maule A, White RF, Cirillo J, Sisson E, Heeren T, Sullivan K, 2017. Neuropsychological characteristics of Gulf War illness: A meta-analysis. PLoS ONE 12, e0177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janulewicz PA, Seth RK, Carlson JM, Ajama J, Quinn E, Heeren T, Klimas N, Lasley SM, Horner RD, Sullivan K, Chatterjee S, 2019. The Gut-Microbiome in Gulf War Veterans: A Preliminary Report. Int. J. Environ. Res. Public Health 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanneteau F, Deinhardt K, Miyoshi G, Bennett AM, Chao MV, 2010. The MAP kinase phosphatase MKP-1 regulates BDNF-induced axon branching. Nat. Neurosci. 13, 1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CS, Bahl JM, Østergaard LB, Høgh P, Wermuth L, Heslegrave A, Zetterberg H, Heegaard NHH, Hasselbalch SG, Simonsen AH, 2019. Exercise as a potential modulator of inflammation in patients with Alzheimer’s disease measured in cerebrospinal fluid and plasma. Exp. Gerontol. 121, 91–98. [DOI] [PubMed] [Google Scholar]
- Kannangara TS, Lucero MJ, Gil-Mohapel J, Drapala RJ, Simpson JM, Christie BR, van Praag H, 2011. Running reduces stress and enhances cell genesis in aged mice. Neurobiol. Aging 32, 2279–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly ÁM, 2018. Exercise-Induced Modulation of Neuroinflammation in Models of Alzheimer’s Disease. Brain Plast. (Amsterdam, Netherlands) 4, 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M, Attaluri S, Madhu LN, Shuai B, Upadhya R, Gonzalez JJ, Rao X, Shetty AK, 2021. Metformin treatment in late middle age improves cognitive function with alleviation of microglial activation and enhancement of autophagy in the hippocampus. Aging Cell 20 (2), e13277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M, Hattiangady B, Shetty GA, Bates A, Shuai B, Shetty AK, 2018. Curcumin treatment leads to better cognitive and mood function in a model of Gulf War Illness with enhanced neurogenesis, and alleviation of inflammation and mitochondrial dysfunction in the hippocampus. Brain Behav. Immun. 69, 499–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M, Megahed T, Mishra V, Shuai B, Hattiangady B, Shetty AK, 2016. Voluntary Running Exercise-Mediated Enhanced Neurogenesis Does Not Obliterate Retrograde Spatial Memory. J. Neurosci. Off. J. Soc. Neurosci. 36, 8112–8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodali M, Parihar VK, Hattiangady B, Mishra V, Shuai B, Shetty AK, 2015. Resveratrol prevents age-related memory and mood dysfunction with increased hippocampal neurogenesis and microvasculature, and reduced glial activation. Sci. Rep. 5, 8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Rhodes JS, 2013. Neurogenesis, inflammation and behavior. Brain Behav. Immun. 27, 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH, Ho TJ, Kuo WW, Day CH, Pai PY, Chung LC, Liao PH, Lin FH, Wu ET, Huang CY, 2014. Exercise training enhanced SIRT1 longevity signaling replaces the IGF1 survival pathway to attenuate aging-induced rat heart apoptosis. Age (Dordrecht, Netherlands) 36, 9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Yau SY, Lee TMC, Lau BW, So KF, 2016. Voluntary Wheel Running Reverses the Decrease in Subventricular Zone Neurogenesis Caused by Corticosterone. Cell Transplant. 25, 1979–1986. [DOI] [PubMed] [Google Scholar]
- Li X, Spence JS, Buhner DM, Hart J Jr., Cullum CM, Biggs MM, Hester AL, Odegard TN, Carmack PS, Briggs RW, Haley RW, 2011. Hippocampal dysfunction in Gulf War veterans: investigation with ASL perfusion MR imaging and physostigmine challenge. Radiology 261, 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian H, Yang L, Cole A, Sun L, Chiang AC, Fowler SW, Shim DJ, Rodriguez-Rivera J, Taglialatela G, Jankowsky JL, Lu HC, Zheng H, 2015. NFκB-activated astroglial release of complement C3 compromises neuronal morphology and function associated with Alzheimer’s disease. Neuron 85, 101–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindheimer JB, Stegner AJ, Wylie GR, Klein-Adams JC, Almassi NE, Ninneman JV, Van Riper SM, Dougherty RJ, Falvo MJ, Cook DB, 2020. Post-exertional malaise in veterans with gulf war illness. Int. J. Psychophysiol. Off. J. Int. Organizat. Psychophysiol. 147, 202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker AR, Michalovicz LT, Kelly KA, Miller JV, Miller DB, O’Callaghan JP, 2017. Corticosterone primes the neuroinflammatory response to Gulf War Illness-relevant organophosphates independently of acetylcholinesterase inhibition. J. Neurochem. 142, 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhu LN, Attaluri S, Kodali M, Shuai B, Upadhya R, Gitai D, Shetty AK, 2019. Neuroinflammation in Gulf War Illness is linked with HMGB1 and complement activation, which can be discerned from brain-derived extracellular vesicles in the blood. Brain Behav. Immun. 81, 430–443. [DOI] [PubMed] [Google Scholar]
- Madhu LN, Kodali M, Attaluri S, Shuai B, Melissari L, Rao X, Shetty AK, 2021. Melatonin improves brain function in a model of chronic Gulf War Illness with modulation of oxidative stress, NLRP3 inflammasomes, and BDNF-ERK-CREB pathway in the hippocampus. Redox Biol. 43, 101973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecca C, Giambanco I, Donato R, Arcuri C, 2018. Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes. Int. J. Mol. Sci. 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee-Inta O, Zhao ZW, Kuo YM, 2019. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon PM, Nasrallah HA, Reeves RR, Ali JA, 2004. Hippocampal dysfunction in Gulf War Syndrome. A proton MR spectroscopy study. Brain Res. 1009, 189–194. [DOI] [PubMed] [Google Scholar]
- Merali Z, Michaud D, McIntosh J, Kent P, Anisman H, 2003. Differential involvement of amygdaloid CRH system(s) in the salience and valence of the stimuli. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 27, 1201–1212. [DOI] [PubMed] [Google Scholar]
- Miller JV, LeBouf RF, Kelly KA, Michalovicz LT, Ranpara A, Locker AR, Miller DB, O’Callaghan JP, 2018. The Neuroinflammatory Phenotype in a Mouse Model of Gulf War Illness is Unrelated to Brain Regional Levels of Acetylcholine as Measured by Quantitative HILIC-UPLC-MS/MS. Toxicol. Sci. Off. J. Soc. Toxicol. 165, 302–313. [DOI] [PubMed] [Google Scholar]
- Nichol KE, Poon WW, Parachikova AI, Cribbs DH, Glabe CG, Cotman CW, 2008. Exercise alters the immune profile in Tg2576 Alzheimer mice toward a response coincident with improved cognitive performance and decreased amyloid. J. Neuroinflamm. 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan JP, Kelly KA, Locker AR, Miller DB, Lasley SM, 2015. Corticosterone primes the neuroinflammatory response to DFP in mice: potential animal model of Gulf War Illness. J. Neurochem. 133, 708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odegard TN, Cooper CM, Farris EA, Arduengo J, Bartlett J, Haley R, 2013. Memory impairment exhibited by veterans with Gulf War Illness. Neurocase 19, 316–327. [DOI] [PubMed] [Google Scholar]
- Parihar VK, Hattiangady B, Shuai B, Shetty AK, 2013. Mood and memory deficits in a model of Gulf War illness are linked with reduced neurogenesis, partial neuron loss, and mild inflammation in the hippocampus. Neuropsychopharmacol. Off. Public. Am. Coll. Neuropsychopharmacol. 38, 2348–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedruzzi LM, Stockler-Pinto MB, Leite M Jr., Mafra D, 2012. Nrf2-keap1 system versus NF-κB: the good and the evil in chronic kidney disease? Biochimie 94, 2461–2466. [DOI] [PubMed] [Google Scholar]
- Peng L, Bonaguidi MA, 2018. Function and Dysfunction of Adult Hippocampal Neurogenesis in Regeneration and Disease. The American journal of pathology 188, 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce C, Audiffren M, 2011. Does acute exercise switch off switch costs? A study with younger and older athletes. J. Sport Exerc. Psychol. 33, 609–626. [DOI] [PubMed] [Google Scholar]
- Piao CS, Stoica BA, Wu J, Sabirzhanov B, Zhao Z, Cabatbat R, Loane DJ, Faden AI, 2013. Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiol. Disease 54, 252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KF, Deshpande LS, 2016. Repeated low-dose organophosphate DFP exposure leads to the development of depression and cognitive impairment in a rat model of Gulf War Illness. Neurotoxicology 52, 127–133. [DOI] [PubMed] [Google Scholar]
- Radak Z, Taylor AW, Ohno H, Goto S, 2001. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc. Immunol. Rev. 7, 90–107. [PubMed] [Google Scholar]
- Radiske A, Rossato JI, Gonzalez MC, Köhler CA, Bevilaqua LR, Cammarota M, 2017. BDNF controls object recognition memory reconsolidation. Neurobiol. Learn. Mem. 142 (Pt A), 79–84. [DOI] [PubMed] [Google Scholar]
- Rao MS, Hattiangady B, Shetty AK, 2008. Status epilepticus during old age is not associated with enhanced hippocampal neurogenesis. Hippocampus 18, 931–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MS, Shetty AK, 2004. Efficacy of doublecortin as a marker to analyze the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur. J. Neurosci. 19, 234–246. [DOI] [PubMed] [Google Scholar]
- Rayhan RU, Stevens BW, Raksit MP, Ripple JA, Timbol CR, Adewuyi O, VanMeter JW, Baraniuk JN, 2013. Exercise challenge in Gulf War Illness reveals two subgroups with altered brain structure and function. PLoS ONE 8, e63903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig JM, Lei J, Burd I, 2014. Interleukin-1 receptor blockade in perinatal brain injury. Front. Pediatr. 2, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SM, Kelly ÁM, 2016. Exercise as a pro-cognitive, pro-neurogenic and anti-inflammatory intervention in transgenic mouse models of Alzheimer’s disease. Age. Res. Rev. 27, 77–92. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Attaluri S, Kodali M, Shuai B, Shetty GA, Upadhya D, Hattiangady B, Madhu LN, Upadhya R, Bates A, Rao X, 2020. Monosodium luminol reinstates redox homeostasis, improves cognition, mood and neurogenesis, and alleviates neuro- and systemic inflammation in a model of Gulf War Illness. Redox Biol. 28, 101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Zanirati G, 2020. The Interstitial System of the Brain in Health and Disease. Aging Disease 11, 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty GA, Hattiangady B, Upadhya D, Bates A, Attaluri S, Shuai B, Kodali M, Shetty AK, 2017. Chronic Oxidative Stress, Mitochondrial Dysfunction, Nrf2 Activation and Inflammation in the Hippocampus Accompany Heightened Systemic Inflammation and Oxidative Stress in an Animal Model of Gulf War Illness. Front. Mol. Neurosci. 10, 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA, 2011. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature 476, 458–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele L, Sastre A, Gerkovich MM, Cook MR, 2012. Complex factors in the etiology of Gulf War illness: wartime exposures and risk factors in veteran subgroups. Environ. Health Perspect. 120, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steventon JJ, Foster C, Furby H, Helme D, Wise RG, Murphy K, 2020. Hippocampal blood flow is increased after 20 min of moderate-intensity exercise. Cereb. Cortex 30, 525–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson M, Rosvall P, Boza-Serrano A, Andersson E, Lexell J, Deierborg T, 2016. Forced treadmill exercise can induce stress and increase neuronal damage in a mouse model of global cerebral ischemia. Neurobiol. Stress 5, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I, 2001. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J. Neurosci. Off. J. Soc. Neurosci. 21, 1628–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitkanou S, Della Gatta P, Foletta V, Russell A, 2019. The Role of Exercise as a Non-pharmacological Therapeutic Approach for Amyotrophic Lateral Sclerosis: Beneficial or Detrimental? Front. Neurol. 10, 783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH, 1999a. Running enhances neurogenesis, learning, and long-term potentiation in mice. PNAS 96, 13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH, 1999b. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270. [DOI] [PubMed] [Google Scholar]
- von Holstein-Rathlou S, Petersen NC, Nedergaard M, 2018. Voluntary running enhances glymphatic influx in awake behaving, young mice. Neurosci. Lett. 662, 253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Klakotskaia D, Ajit D, Weisman GA, Wood WG, Sun GY, Serfozo P, Simonyi A, Schachtman TR, 2015. Beneficial effects of dietary EGCG and voluntary exercise on behavior in an Alzheimer’s disease mouse model. J. Alzheimer’s Dis. : JAD 44, 561–572. [DOI] [PubMed] [Google Scholar]
- White RF, Steele L, O’Callaghan JP, Sullivan K, Binns JH, Golomb BA, Bloom FE, Bunker JA, Crawford F, Graves JC, Hardie A, Klimas N, Knox M, Meggs WJ, Melling J, Philbert MA, Grashow R, 2016. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex: J. Devot. Study Nerv. Syst. Behav. 74, 449–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Melnik A, Kempermann G, 2011. Physical exercise increases adult neurogenesis and telomerase activity, and improves behavioral deficits in a mouse model of schizophrenia. Brain Behav. Immun. 25, 971–980. [DOI] [PubMed] [Google Scholar]
- Woost L, Bazin PL, Taubert M, Trampel R, Tardif CL, Garthe A, Kempermann G, Renner U, Stalla G, Ott DVM, Rjosk V, Obrig H, Villringer A, Roggenhofer E, Klein TA, 2018. Physical Exercise and Spatial Training: A Longitudinal Study of Effects on Cognition, Growth Factors, and Hippocampal Plasticity. Sci. Rep. 8, 4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM, 2013. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 18, 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie GR, Genova H, Dobryakova E, DeLuca J, Chiaravalloti N, Falvo M, Cook D, 2019. Fatigue in Gulf War Illness is associated with tonically high activation in the executive control network. NeuroImage. Clinical 21, 101641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark CE, 2011. Pattern separation in the hippocampus. Trends Neurosci. 34, 515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakirova Z, Crynen G, Hassan S, Abdullah L, Horne L, Mathura V, Crawford F, Ait-Ghezala G, 2015. A Chronic Longitudinal Characterization of Neurobehavioral and Neuropathological Cognitive Impairment in a Mouse Model of Gulf War Agent Exposure. Front. Integr. Neurosci. 9, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]


