Figure 1.
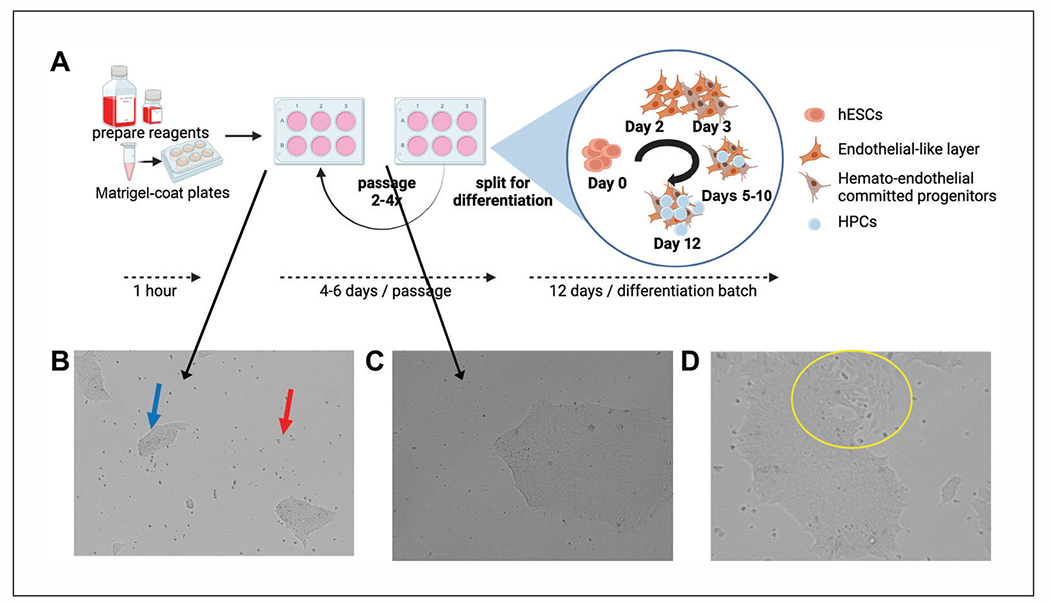
Maintenance of hESCs. (A) Overview of hESC maintenance and differentiation experiments with estimated timing. Reagents for maintenance and differentiation require thawing overnight at 4°C. hESC should be passaged at least twice after thawing and recovery prior to beginning a differentiation experiment. A normal passaging period for healthy WA01 or WA09 cells split at an average colony size with ~10% initial confluence equivalency should be passaged every 4 to 6 days. Note that aggregates grow exponentially faster at later days post-split and can easily become overcrowded. Differentiation of hESCs to HPCs requires 12 days using this method and begins with aggregate plating, as for passaging, followed by incubation in specific differentiation media that induce hemato-endothelial differentiation that mimics embryonic development from aggregate expansion and endothelial differentiation to hematopoietic development in layers on top of the adherent endothelial-like cells (more details in Fig. 2). Panel (A) was created with BioRender.com. (B) WA01 hESCs at passage 30 were cultured and maintained in mTeSR1. The image shows a representative view of hESCs at 1 day post-split where both adherent aggregates (blue arrow) and some floating cells (red arrow) are present prior to medium exchange. (C) A single aggregate of WA01 cells from a low-density split at 5 days and ready for passage. Note that although aggregates are not at 90% confluence equivalency, individual aggregates are large and dense. (D) WA09 (passage 34) hESCs, which were cultured as in (B), showing signs of spontaneous differentiation (yellow circle) based on morphological changes among individual cells at the edges of the aggregate and changes in overall aggregate-edge morphology.
