Abstract
Novelty seeking is a tendency to approach new situations, putatively driven by the brain’s catecholaminergic system. It is traditionally measured via self-report, but a laboratory-based paradigm, the human Behavioral Pattern Monitor (hBPM), quantifies behavior in a novel environment and has utility in cross-species studies of neuropsychiatric disorders. Our primary aim assessed whether self-reported novelty-seeking traits were associated with novelty-seeking behavior in the hBPM. An existing sample of 106 volunteers were categorized as high vs. low novelty seekers using the Temperament and Character Inventory (TCI). Subjects had been randomized to one dose of amphetamine (10 or 20 mg) or modafinil (200 or 400 mg), allowing us to explore whether a pharmacological catecholamine challenge further enhanced novelty-seeking behavior. High TCI novelty-seekers had more hBPM motor activity and novel object interactions. The exploratory analyses, although limited by low power, suggested that amphetamine and modafinil did not markedly moderate novelty-seeking traits. The hBPM demonstrates construct validity as a lab-based measure of novelty seeking and thus useful in translational studies of neuropsychiatric conditions and treatment options. Further research may illuminate whether a biological predisposition towards higher catecholaminergic activity, combined with the novelty-seeking trait, may increase propensity for risky and addictive behaviors.
Keywords: Temperament and Character Inventory, motor activity, exploration, amphetamine, modafinil
1. Introduction
Novelty seeking is a trait, defined as a proclivity to approach unfamiliar stimuli or situations, and in its exaggerated form reflects a decreased ability to self-regulate behavior. It is thought to be a moderately heritable trait, with heritability estimates ranging from .28 to .36 in a twin study (Heiman et al., 2004). High novelty seeking, traditionally measured with self-report indices of personality features and historical behavior, is a major predictor of impulsive, addictive behaviors as well as activities associated with personal risk (Golimbet et al., 2007; Zald et al., 2008). Exaggerated novelty seeking may underlie some cardinal symptoms of psychiatric conditions such as bipolar disorder (BD), Attention-Deficit Hyperactivity Disorder (ADHD), substance use disorders, and other pathologies.
Novelty seeking is a phenotype that occurs across species, thus, its quantification can assist in validating animal models of neuropsychiatric disorders which can in turn advance drug discovery. A behavioral paradigm that has been reverse-translated from animals to humans is the Behavioral Pattern Monitor (BPM), which quantifies an organism’s behavior in a novel environment. We have previously used the human (h)BPM and its animal analog to describe unique and signature patterns of abnormal behavior in psychiatric conditions and following pharmacological challenges across species. For example, in patients both in the manic (Minassian et al., 2010; Minassian et al., 2011; Perry et al., 2010; Perry et al., 2009) and stabilized euthymic phases (Henry et al., 2013b) of BD, high motor activity, increased novel object interaction, and straight and expansive movements are observed. This pattern was recreated in two mouse models of mania of reduced dopamine (DA) transporter function (Perry et al., 2009; Young et al., 2010). This BD signature can be differentiated from hBPM behavior in people with schizophrenia, characterized by reduced habituation and straight-line movement without novel object seeking, people with methamphetamine dependence, characterized solely by high object interactions (Henry et al., 2011), and distinct from acute amphetamine administration in healthy humans which causes elevations in motor activity only (Minassian et al., 2016).
A recent large study has reported that novelty-seeking traits measured in adolescents are associated with specific brain areas involved in reward (Qi et al., 2021) which ultimately predicted increased risk for addiction behaviors, depression, and psychosis five years later. That study provides new and compelling evidence for the important role of this phenotype with respect to perturbations in the brain’s reward system and for conferring risk for pathology. To that end, we used an existing dataset to study several to-date unanswered questions.
While we have conceptualized the hBPM as useful in quantifying novelty-seeking behavior, it would be important to test whether novelty-seeking traits as traditionally measured by self-report of personality features and historical behavior manifest in novelty-seeking behavior in an experimental, quantifiable, lab-based paradigm. We used the gold standard measure for measuring this trait, the Temperament and Character Inventory (TCI) (Cloninger et al., 1993). We hypothesized that the trait of novelty-seeking as measured by the TCI would be associated with increased novelty-seeking behavior in the hBPM. If this hypothesis were supported, it would provide construct validity for the hBPM paradigm and its utility in measuring this trans-diagnostic phenotype.
The neurotransmitters most closely linked to novelty-seeking traits and behavior are the catecholamines (CAs) DA and norepinephrine (NE). Novelty seeking is theoretically driven by the brain’s reward system, as the discovery of a novel stimulus reinforces further exploration for novelty (Krebs et al., 2009). DA is thought to play a vital role in reinforcement and reward (Jentsch et al., 2000; Schultz, 1998), and NE has been linked to the traits of novelty seeking (Lee et al., 2008), sensation seeking (Roy et al., 1988) and reward dependence (Garvey et al., 1996). Neuroimaging (Bunzeck and Duzel, 2006; Cohen et al., 2009) and genetic (Golimbet et al., 2007) studies also implicate DA pathways in novelty seeking.
The subjects in our existing dataset were enrolled in a stimulant challenge study where they received a one-time dose of either d-amphetamine or modafinil. Thus, as a secondary question we explored whether pharmacological agonism of the CA system contributes to novelty-seeking behaviors in the hBPM. We predicted that administration of CA transporter inhibitors/DA releasers such as d-amphetamine and modafinil would also increase novelty-seeking compared to placebo-treated participants, particularly in individuals with the high novelty-seeking trait. These questions were exploratory given the limited sample size for these secondary analyses, but were hoped to provide initial data for more systematic investigations in the future.
2. Method
2.1. Participants
We performed retrospective analyses on an existing sample of 106 healthy volunteers. These series of experiments were part of a larger study investigating the effect of dopamine agonists on inhibition, exploratory behavior, and cognition (R01 MH071916). All participants were administered the TCI prior to administration of the drug. Following TCI administration, subjects were randomized to a single dose of placebo, 10 or 20 mg d-amphetamine, or 200 or 400 mg modafinil, with an original randomization scheme of 2:1 placebo vs. each active drug condition, and tested in the hBPM. The doses of amphetamine were based upon those used in previous studies (Ballard et al., 2014; de Wit et al., 2002; Weafer and de Wit, 2013); the 10- and 20-mg doses have elicited behavioral effects and have been generally well-tolerated in those reports. We have previously published that the 20-mg dose increased motor activity in this cohort of subjects as well as in wildtype mice (Minassian et al., 2016). The 200 mg and 400 mg doses of modafinil were selected because 200 mg is a typical starting clinically therapeutic dose, and 400 mg has been shown to affect risk-taking behavior in healthy adults (Killgore et al., 2008). We have previously published that these doses had a beneficial effect on attention in this cohort of subjects (Cope et al., 2017).
The University of California San Diego (UCSD) School of Medicine’s institutional review board approved the study. Participants were recruited from flyers and on-line advertisements posted in the community and included 106 male and female volunteers with the following inclusion criteria: 1. Ages 18-35. 2. In good general health. 3. No lifetime history of an Axis I or Axis II disorder. 4. No first-degree relative with a history of psychotic or mood disorder. 5. No specific contraindications or previous adverse reactions to amphetamine or modafinil. Exclusion criteria included: 1. Clinically significant electrocardiogram or physical exam findings as determined by the study physician. 2. Women with a positive serum HCG pregnancy test or who are lactating. 3. History of alcohol or substance (e.g., sedative-hypnotics, cannabis, stimulants, opioids, cocaine, hallucinogens) abuse or dependence within the last 30 days, or a positive result on a urine toxicology screen for illegal substances completed on study entrance. Nicotine abuse or dependence was not an exclusion criterion. 4. Current severe, systemic medical illness that may compromise cognitive functioning or serious cardiac disease. 5. Current or history of neurological disorder such as seizures or stroke, Parkinson’s Disease, dementia, or a history of head injury with loss of consciousness for at least 15 min.
At study’s end and final unblinding, 33 subjects had been randomized to placebo, 18 subjects to amphetamine 10 mg, 26 subjects to amphetamine 20 mg, 14 subjects to modafinil 200 mg, and 15 subjects to modafinil 400 mg. The sample size for the 10-mg dose of amphetamine and the 200 and 400 mg doses of modafinil are lower than that of the 20 mg dose because an interim unblinding and data analysis indicated no trends for effects of those drugs. In an effort to complete data collection in a timely manner randomization into those drugs conditions were stopped.
2.2. Procedure
After providing informed consent, participants underwent a physical examination by an MD and were administered an ECG. Clinically significant findings on these exams constituted exclusion from the study (n=5). They were then assessed with the SCID to rule out Axis I and Axis II disorders. They were asked for a urine sample for toxicology analysis and pregnancy test if applicable.
The TCI-125 (Cloninger et al., 1994) was administered to participants prior to administration of the drug. The TCI and its predecessor the Tridimensional Personality Questionnaire have been used extensively in genetic studies of novelty seeking in relation to CA-related genes (Demetrovics et al., 2010; Lee et al., 2010; Okuyama et al., 2000; Sadahiro et al., 2010; Staner et al., 1998). The TCI-125 consists of 125 true/false statements and participants are asked to respond based on how they usually act or feel and not just how they are feeling right now; for example “I often try new things just for fun or thrills, even if most people think it is a waste of time”. Statements endorsed as “true” are parsed into 5 subscales including the Novelty Seeking subscale which was the focus of this investigation (other subscales include Harm Avoidance, Reward Dependence, Persistence, Self-Directiveness, Cooperativeness, and Self-Transcendence). Higher scores on the subscale reflect a stronger trait.
Randomization and administration of study drug was conducted by the UCSD Investigational Pharmacy. Participants entered the hBPM 60 min after administration of study drug. This time interval was largely based upon the pharmacokinetic profile of amphetamine and evidence that it reaches peak plasma concentrations at 2-2.5 hr post-ingestion (Wong et al., 1998) and broadly consistent with other studies on the behavioral effects of d-amphetamine (60 min-90 min) (Ballard et al., 2014; de Wit et al., 2002; Weafer and de Wit, 2013). Assessment of vital signs (heart rate and blood pressure) was conducted prior to ingestion of study drug and immediately prior to the end of the entire test battery. Since physiologic parameters can be impacted by body weight, height and weight were collected for the calculation of Body Mass Index (BMI).
Prior to entering the hBPM, participants were fitted with an ambulatory monitoring device worn on the torso that quantifies motor activity via triaxial accelerometer output (Hidalgo, 2010; Vivometrics, 2002). Encrypted data were stored on a removable memory card and subsequently extracted and analyzed with the Vivosense™ proprietary PC-based software (version 2.7). Mean acceleration values were generated from a filtered summation of movement on the x, y, and z axes while incorporating force effects due to gravity. After being fit with the monitor, individuals were placed in the hBPM for 15 min without explicit directions except to wait for the experimenter to prepare another task.
The hBPM has been described previously (Henry et al., 2013b; Henry et al., 2011; Minassian et al., 2010; Minassian et al., 2011; Perry et al., 2010; Perry et al., 2009). The hBPM (Figure 1) consists of a 3.5 m by 4.9 m rectangular room that contains several items of furniture, including two bookcases, several tall filing cabinets, a corkboard mounted on the wall, and a short storage chest, but no chairs. A number of small objects were placed throughout the room to stimulate exploration and invite participant interaction (Henry et al., 2011; Minassian et al., 2011; Perry et al., 2009). These items were selected to meet several criteria, including being safe, colorful, tactile, and manipulable (Pierce and Courchesne, 2001); they include a feather mask that could be worn, a kaleidoscope, finger puppets, and a paddle ball game. Participant activity in the room was monitored and recorded by a camera concealed in the ceiling that is equipped with a fish-eye lens capable of viewing the entire room. Digitized videos sampled at 30 frames per second were stored on a computer in an adjacent room for subsequent analysis (Perry et al., 2010).
Figure 1.
The human Behavioral Pattern Monitor (hBPM)
During the informed consent process, subjects were told that they might be videotaped during a part of their examination, but were not specifically instructed that this recording would occur during the hBPM session. Following the hBPM session, subjects were returned to the laboratory where additional testing was completed.
2.3. Data Analysis and Statistics
In order to reduce Type I error, dependent variables were limited to two hBPM variables that have yielded most pronounced group differences in our previous work and have the most conceptual convergence with the phenotype of novelty seeking: motor activity as measured by acceleration, and number of novel object interactions.
To assess motor activity, mean acceleration in digital units was derived for each of the three 5-min epochs of the 15-min hBPM session; mean values of acceleration are a common and accepted method with which to determine static and dynamic motor activity (Godfrey et al., 2008). We have found previously that there can be changes in hBPM activity as the 15-minute session progresses. For example our initial study in manic BD subjects suggested a significant decrease in acceleration and object interactions from the first or second 5-minute epoch of the hBPM to the last (Perry et al., 2009); thus epoch is typically included as a repeated measure in hBPM analyses.
Object interactions in the hBPM were quantified manually by trained raters blind to group condition who evaluated participant exploration in one-second increments during the video recording. We have previously established interrater reliability for hBPM video ratings; kappa reliability coefficients for rater-coded measures range from 0.91 to 0.96 (Perry et al., 2010). We quantified the total number of object interactions, defined as deliberate physical contact with a novel object with any part of the body, e.g., hand or foot. Again, object interactions for each of the three 5-minute epochs of the hBPM were quantified.
Participants were grouped based on whether (high novelty seeking) or not (low novelty seeking) their TCI Novelty Seeking Subscale scores were in the top 40% of the sample or not, respectively. Whereas the Qi et al study characterized the top 20% of adolescents as high in the novelty-seeking trait, the current study has a much smaller sample size, and a 40% cutoff allowed us to capture those with the highest scores while retaining a sufficient sample size for the high novelty-seeking group.
Statistical analysis:
To assess the relationship between novelty-seeking traits and hBPM activity, we conducted repeated measures 3x2 analyses of variance (ANOVAs), with the three 5-minute epochs of the hBPM as the repeated measure, and novelty-seeking group as the between-subjects factor. Additionally, Pearson r correlation coefficients were generated on the entire available sample between the TCI novelty-seeking score and the hBPM measures.
For the exploratory questions on the effects of stimulants on novelty seeking, we conducted repeated-measures 3 x 2 x 5 ANOVAs, with novelty-seeking group and the 5 drug groups as between-subjects factors.
Significance values were set at p < .05. Partial eta-squared (pη2) was used for effect sizes in the ANOVAs (.01=small effect, .06=medium effect, .14= large effect) (Stevens, 1992). In analyses where effect sizes were at least medium but statistical significance was not achieved, observed power for the analysis is reported.
Outliers were considered those hBPM values greater than 2 standard deviations from the grand mean. Four subjects had outlier data for at least one of the acceleration epochs, and they were not included in the acceleration analyses. Six subjects had outlier data for at least one of the object interaction epochs and were not included in the object interaction analyses.
3. Results
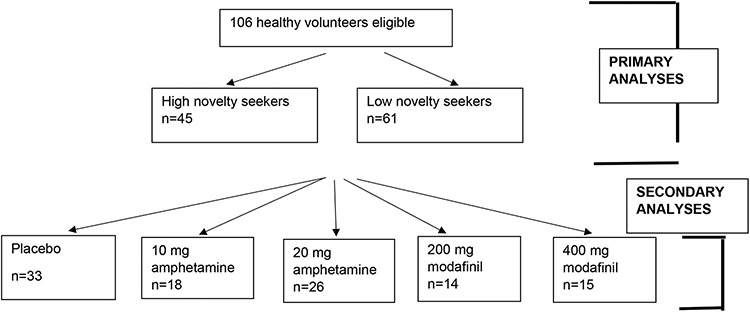
A participant flow diagram can be found in Figure 2. Demographic information can be found in Table 1. The two novelty-seeking groups were well-matched on most demographics. Demographic information for the drug groups can be found in Supplementary Materials Table S1.
Figure 2.
Participant Flow Diagram.
Table 1.
Participant demographic information.
| High novelty-seekers (n=45) |
Low novelty-seekers (n=61) |
Group difference |
|
|---|---|---|---|
| Age in years | 23.2 (4.3) | 24.0 (4.7) | ns |
| Gender | 23 F, 22 M | 37 F, 24 M | ns |
| Race/Ethnicity | 17 White Non-Hispanic 11 White Hispanic 11 Asian 4 African-American 2 More than one race |
13 White Non-Hispanic 23 White Hispanic 19 Asian 3 African-American 1 American Indian/Alaska Native 2 More than one race |
ns |
| Yrs. education | 15.1 (1.5) | 15.0 (2.2) | ns |
| Body mass index | 24.1 (3.8) | 24.1 (4.6) | ns |
| TCI novelty-seeking subscale score | 12.0 (2.0) | 6.3 (1.7) | F(1,105)=252,6, p < .001 |
Data are presented as means (standard deviations) unless otherwise noted. High novelty-seekers are defined as those who scored in the top 40% of the TCI novelty-seeking subscale. TCI=Temperament and Character Inventory. ns=not statistically significant
3.1. Novelty-seeking traits and hBPM behavior
hBPM Acceleration:
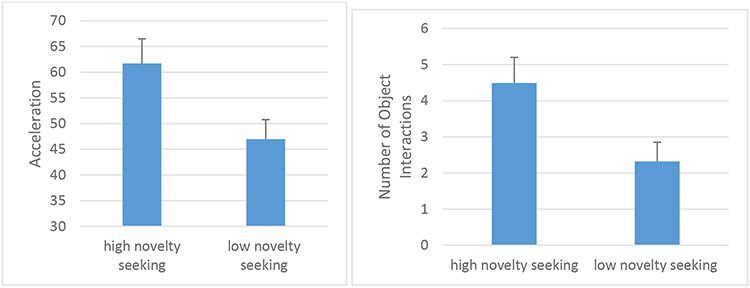
There was a main effect of novelty-seeking group [F(1, 100)=5.67, p=.02, pη2=.05) such that subjects in the high novelty-seeking group had higher acceleration (Figure 3a). There was no effect of hBPM epoch [F(2,200)=.41, ns, pη2=.004] nor an epoch-by-novelty-seeking group interaction [F(2,200)=.19, ns, pη2=.002].
Figure 3.
Higher novelty-seeking scores on the TCI are associated with higher motor activity in the hBPM as measured by acceleration (Fig 3a) and higher number of hBPM novel object interactions (Fig 3b).
hBPM Object Interactions:
There was a main effect of novelty-seeking group [F(1, 98)=5.77, p=.018, pη2=.06] such that subjects in the high novelty-seeking group had more object interactions (Figure 3b). There was an effect of hBPM epoch [F(2,196)=5.66, p=.004, pη2=.06] such that object interactions decreased over the 5-minute epochs, but there was no epoch-by-novelty-seeking group interaction [F(2,196)=1.17, ns, pη2=.01].
3.2. Novelty-seeking traits and impact of drugs that increase DA levels
hBPM Acceleration:
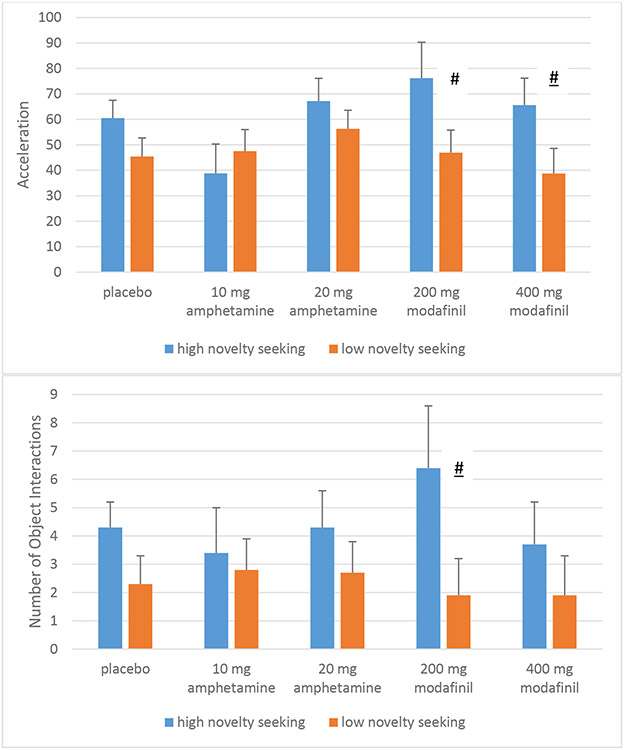
There was no main effect of epoch on acceleration [F(2, 184)=.49, ns, pη2=.01]. As observed in the primary analyses, there was a significant main effect of novelty-seeking group, with a medium effect size [F(1,92)=5.8, p=.02, pη2=.06] such that high novelty-seekers had more acceleration. There was no main effect of drug [F(4,92)=1.3, ns, pη2=.05, observed power= .38], although the effect size was medium, and post-hoc group comparisons indicated that 20 mg d-amphetamine was associated with higher acceleration compared to 10 mg d-amphetamine (p=.046), consistent to what we have reported previously (Minassian et al., 2016). There was no drug x novelty-seeking group interaction [F(4,92)=1.0, ns, pη2=.044]. Pairwise comparisons suggested a trend of higher acceleration in high novelty-seekers given modafinil compared to low novelty-seekers (Figure 4a).
Figure 4.
Novelty seeking on the TCI is not strongly associated with catecholamine agonist drug effects in the hBPM as measured by acceleration (Figure 4a) and number of hBPM novel object interactions (Figure 4b).
Figure legend: # High novelty-seekers > low novelty-seekers, p < .10.
hBPM Object Interactions:
There was no main effect of epoch on object interactions [F(2, 180)=.2.91, ns, pη2=.03]. As observed in the primary analyses, there was a main effect of novelty-seeking group, with a medium effect size [F(1,90)=5.7, p=.02, pη2=.06] such that higher novelty-seekers had more object interactions. There was no main effect of drug [F(4,90)=.19, ns, pη2=.01] and no drug x novelty-seeking group interaction [F(4,89)=.38, ns, pη2=.017]. Pairwise comparisons suggested a trend of higher object interactions in high novelty-seekers given 200 mg modafinil compared to low novelty-seekers given the same dose (Figure 4b).
3.3. Relationship between novelty-seeking traits and hBPM variables
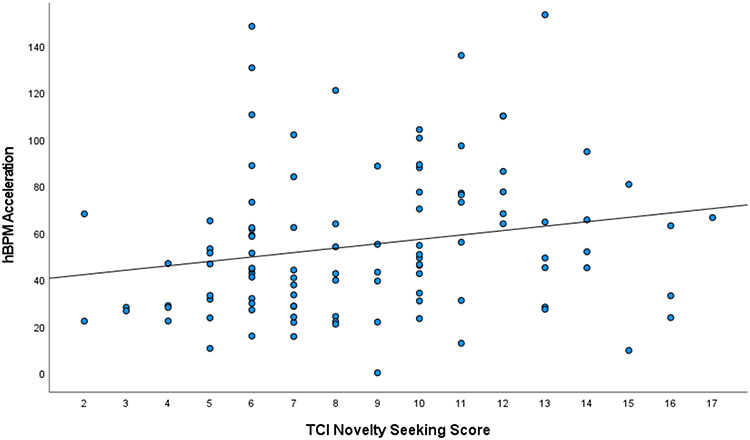
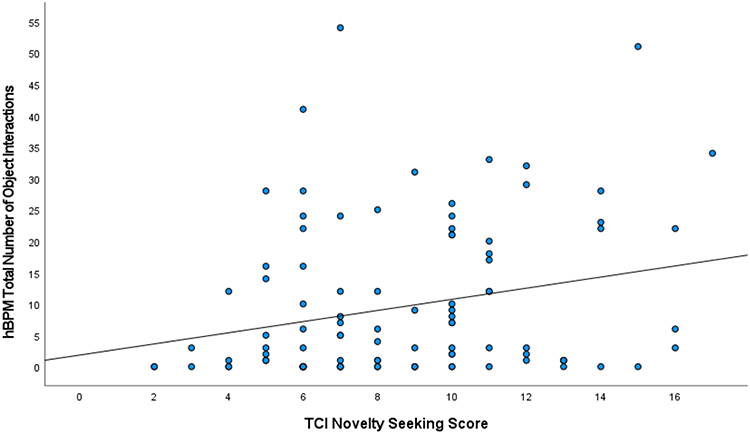
Pearson r correlations can be found in Table 2. Supporting the high versus low novelty-seeking group differences on hBPM activity, higher novelty-seeking scores on the TCI were positively correlated with acceleration and total object interactions (Figure 5).
Table 2.
Pearson r correlation coefficients for TCI and hBPM measures.
| TCI novelty- seeking score |
|
|---|---|
| Acceleration | .20 (p=.04) |
| Object interactions | .25 (p=.01) |
TCI=Temperament and Character Inventory
Figure 5.
Scatterplot of correlation between TCI novelty-seeking score and motor activity in the hBPM as measured by acceleration (Fig 5A), and TCI novelty-seeking score and hBPM novel object interactions (Fig 5B).
Discussion
The trait of novelty seeking can be predictive of engagement in high-risk behavior and has been associated with addictive disorders as well as neuropsychiatric conditions such as BD among others. More recently, novelty seeking predict abnormalities in brain reward circuitry and ultimately risk of addiction, depression, and psychosis (Qi et al., 2021). Given the relevance of this phenotype in CA-mediated conditions, we used a convenience-sample of healthy volunteers to query whether self-report of novelty-seeking traits are associated with novelty-seeking behavior in an experimental paradigm, the hBPM. This relationship was supported by our findings. Our exploratory analyses suggested that a single dose of drugs that increase CA levels did not markedly interact with novelty-seeking traits to influence behavior in the hBPM.
Increased activity in the hBPM as indexed by quantity of motor activity and particularly by interaction with novel objects was reliably higher in the subjects who had high (top 40%) scores on the novelty-seeking subscale of the TCI. Additionally, scores on this subscale were positively correlated with the hBPM motor activity and object interaction variables. These findings provide construct validity for the hBPM as a method as a laboratory-based quantification of novelty seeking, which underscores its utility in studying this phenotype. We have reported that populations with putative dopaminergic dysregulation, such as those with BD (during periods of euthymia and mania), and people with methamphetamine dependence, demonstrate increased engagement with novel objects in the hBPM (Henry et al., 2013b; Henry et al., 2011; Minassian et al., 2011; Perry et al., 2009). The novelty-seeking phenotype is also observed in mouse models of mania and to some extent a mouse model of heavy methamphetamine use (Henry et al., 2013a). Validating measures of phenotypes that have cross-species relevance, such as novelty seeking, supports the utility of preclinical research and the validation of animal models to study neuropsychiatric conditions and guide drug discovery (Geyer, 2008). The validation of this task for use in rodents also enables testing related mechanisms, such as the evidence that pharmacological reduction of dopamine levels in mice with reduced DA transporter expression normalized their novelty seeking behavior (van Enkhuizen et al., 2014).
Whereas we had predicted that a CA agonist in combination with the novelty-seeking trait would have an additive effect on novelty-seeking behavior, we did not observe this effect with a single dose of either amphetamine or modafini, though some weak trends in that direction were observed for subjects who were randomized to modafinil. These exploratory analyses had significant limitations, e.g., small sample sizes and insufficient power to achieve statistical significance, particularly when testing interactions. Post-hoc power estimates confirmed low power in the analyses that included drug group. Some moderate effect sizes were observed, but we recognize that underpowered studies may overestimate effect magnitude (Button et al., 2013). Importantly, a single administration of agents increasing catecholamine levels by amphetamine and modafinil may have been insufficient to yield an additive effect in the analyses presented here. It is unknown whether more prolonged exposure to elevated catecholaminergic levels, as would be the case for people with a substance use disorder, may have magnified this effect.
In conclusion, we have demonstrated construct validity for hBPM measures of the novelty-seeking behavioral phenotype. Self-report of novelty-seeking traits are associated with novelty-seeking behavior in this paradigm. A single dose of a CA agonist did not dramatically magnify novelty-seeking behavior in these individuals who did not have a psychiatric condition or substance dependence. A biological propensity towards overactivation of the brain’s reward system in combination with the novelty-seeking trait may confer high risk for engaging in dangerous behaviors. This study supports the utility of the behavioral pattern monitor in translational studies of neuropsychiatric conditions and drug discovery.
Supplementary Material
Acknowledgment
This study was supported by a NARSAD Young Investigator grant (AM), R01 MH071916 (WP), and R01 DA043535 (WP/JY). The authors thank Tatyana Shekhtman for her assistance.
References
- Ballard ME, Gallo DA, de Wit H, 2014. Amphetamine increases errors during episodic memory retrieval. J Clin Psychopharmacol 34 (1), 85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunzeck N, Duzel E, 2006. Absolute coding of stimulus novelty in the human substantia nigra/VTA. Neuron 51 (3), 369–379. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, Munafo MR, 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci 14 (5), 365–376. [DOI] [PubMed] [Google Scholar]
- Cloninger CR, Przybeck T, Svrakic D, Wetzel R, 1994. The Temperament and Character Inventory: a guide to its development and use. Washington University, Center for Psychobiology of Personality, St. Louis. [Google Scholar]
- Cloninger CR, Svrakic DM, Przybeck TR, 1993. A psychobiological model of temperament and character. Arch Gen Psychiatry 50 (12), 975–990. [DOI] [PubMed] [Google Scholar]
- Cohen MX, Schoene-Bake JC, Elger CE, Weber B, 2009. Connectivity-based segregation of the human striatum predicts personality characteristics. Nat Neurosci 12 (1), 32–34. [DOI] [PubMed] [Google Scholar]
- Cope ZA, Minassian A, Kreitner D, MacQueen DA, Milienne-Petiot M, Geyer MA, Perry W, Young JW, 2017. Modafinil improves attentional performance in healthy, non-sleep deprived humans at doses not inducing hyperarousal across species. Neuropharmacology 125, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB, 2002. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology 27 (5), 813–825. [DOI] [PubMed] [Google Scholar]
- Demetrovics Z, Varga G, Szekely A, Vereczkei A, Csorba J, Balazs H, Hoffman K, Sasvari-Szekely M, Barta C, 2010. Association between Novelty Seeking of opiate-dependent patients and the catechol-O-methyltransferase Val(158)Met polymorphism. Compr Psychiatry 51 (5), 510–515. [DOI] [PubMed] [Google Scholar]
- Garvey MJ, Noyes R Jr., Cook B, Blum N, 1996. Preliminary confirmation of the proposed link between reward-dependence traits and norepinephrine. Psychiatry Res 65 (1), 61–64. [DOI] [PubMed] [Google Scholar]
- Geyer MA, 2008. Developing translational animal models for symptoms of schizophrenia or bipolar mania. Neurotox Res 14 (1), 71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A, Conway R, Meagher D, O.L. G, 2008. Direct measurement of human movement by accelerometry. Med Eng Phys 30 (10), 1364–1386. [DOI] [PubMed] [Google Scholar]
- Golimbet VE, Alfimova MV, Gritsenko IK, Ebstein RP, 2007. Relationship between dopamine system genes and extraversion and novelty seeking. Neurosci Behav Physiol 37 (6), 601–606. [DOI] [PubMed] [Google Scholar]
- Heiman N, Stallings MC, Young SE, Hewitt JK, 2004. Investigating the genetic and environmental structure of Cloninger's personality dimensions in adolescence. Twin Res 7 (5), 462–470. [DOI] [PubMed] [Google Scholar]
- Henry BL, Geyer MA, Buell M, Perry W, Young JW, Minassian A, 2013a. Behavioral effects of chronic methamphetamine treatment in HIV-1 gp120 transgenic mice. Behav Brain Res 236 (1), 210–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, Patt VM, Hua J, Young JW, Geyer MA, Perry W, 2013b. Inhibitory deficits in euthymic bipolar disorder patients assessed in the human behavioral pattern monitor. J Affect Disord 150 (3), 948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry BL, Minassian A, van Rhenen M, Young JW, Geyer MA, Perry W, 2011. Effect of methamphetamine dependence on inhibitory deficits in a novel human open-field paradigm. Psychopharmacology (Berl) 215 (4), 697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo, 2010. Equivital lifemonitor. Cambridgeshire, UK. [Google Scholar]
- Jentsch JD, Roth RH, Taylor JR, 2000. Role for dopamine in the behavioral functions of the prefrontal corticostriatal system: implications for mental disorders and psychotropic drug action. Prog Brain Res 126, 433–453. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Grugle NL, Killgore DB, Leavitt BP, Watlington GI, McNair S, Balkin TJ, 2008. Restoration of risk-propensity during sleep deprivation: caffeine, dextroamphetamine, and modafinil. Aviat Space Environ Med 79 (9), 867–874. [DOI] [PubMed] [Google Scholar]
- Krebs RM, Schott BH, Schutze H, Duzel E, 2009. The novelty exploration bonus and its attentional modulation. Neuropsychologia 47 (11), 2272–2281. [DOI] [PubMed] [Google Scholar]
- Lee BC, Yang JW, Lee SH, Kim SH, Joe SH, Jung IK, Choi IG, Ham BJ, 2008. An interaction between the norepinephrine transporter and monoamine oxidase A polymorphisms, and novelty-seeking personality traits in Korean females. Prog Neuropsychopharmacol Biol Psychiatry 32 (1), 238–242. [DOI] [PubMed] [Google Scholar]
- Lee SY, Chen SL, Chen SH, Huang SY, Tzeng NS, Chang YH, Wang CL, Lee IH, Yeh TL, Yang YK, Lu RB, 2010. The COMT and DRD3 genes interacted in bipolar I but not bipolar II disorder. World J Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Geyer MA, Paulus MP, Young JW, Perry W, 2010. The quantitative assessment of motor activity in mania and schizophrenia. J Affect Disord 120 (1-3), 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Young JW, Masten V, Geyer MA, Perry W, 2011. Repeated assessment of exploration and novelty seeking in the human behavioral pattern monitor in bipolar disorder patients and healthy individuals. PLoS One 6 (8), e24185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Young JW, Cope ZA, Henry BL, Geyer MA, Perry W, 2016. Amphetamine increases activity but not exploration in humans and mice. Psychopharmacology (Berl) 233 (2), 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama Y, Ishiguro H, Nankai M, Shibuya H, Watanabe A, Arinami T, 2000. Identification of a polymorphism in the promoter region of DRD4 associated with the human novelty seeking personality trait. Mol Psychiatry 5 (1), 64–69. [DOI] [PubMed] [Google Scholar]
- Perry W, Minassian A, Henry B, Kincaid M, Young JW, Geyer MA, 2010. Quantifying over-activity in bipolar and schizophrenia patients in a human open field paradigm. Psychiatry Res 178 (1), 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, Ferguson EJ, Henry BL, Zhuang X, Masten VL, Sharp RF, Geyer MA, 2009. A reverse-translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Arch Gen Psychiatry 66 (10), 1072–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Courchesne E, 2001. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biological Psychiatry 49, 655–664. [DOI] [PubMed] [Google Scholar]
- Qi S, Schumann G, Bustillo J, Turner JA, Jiang R, Zhi D, Fu Z, Mayer AR, Vergara VM, Silva RF, Iraji A, Chen J, Damaraju E, Ma X, Yang X, Stevens M, Mathalon DH, Ford JM, Voyvodic J, Mueller BA, Belger A, Potkin SG, Preda A, Zhuo C, Xu Y, Chu C, Banaschewski T, Barker GJ, Bokde ALW, Quinlan EB, Desrivières S, Flor H, Grigis A, Garavan H, Gowland P, Heinz A, Martinot JL, Paillère Martinot ML, Artiges E, Nees F, Orfanos DP, Paus T, Poustka L, Hohmann S, Fröhner JH, Smolka MN, Walter H, Whelan R, Calhoun VD, Sui J, 2021. Reward Processing in Novelty Seekers: A Transdiagnostic Psychiatric Imaging Biomarker. Biol Psychiatry 90 (8), 529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Adinoff B, Roehrich L, Lamparski D, Custer R, Lorenz V, Barbaccia M, Guidotti A, Costa E, Linnoila M, 1988. Pathological gambling. A psychobiological study. Arch Gen Psychiatry 45 (4), 369–373. [DOI] [PubMed] [Google Scholar]
- Sadahiro R, Suzuki A, Shibuya N, Kamata M, Matsumoto Y, Goto K, Otani K, 2010. Association study between a functional polymorphism of tyrosine hydroxylase gene promoter and personality traits in healthy subjects. Behav Brain Res 208 (1), 209–212. [DOI] [PubMed] [Google Scholar]
- Schultz W, 1998. Predictive reward signal of dopamine neurons. J Neurophysiol 80 (1), 1–27. [DOI] [PubMed] [Google Scholar]
- Staner L, Hilger C, Hentges F, Monreal J, Hoffmann A, Couturier M, Le Bon O, Stefos G, Souery D, Mendlewicz J, 1998. Association between novelty-seeking and the dopamine D3 receptor gene in bipolar patients: a preliminary report. Am J Med Genet 81 (2), 192–194. [PubMed] [Google Scholar]
- Stevens J, 1992. Applied Multivariate Statistics for the Social Sciences: Second Edition. Lawrence Erlbaum Associates, Inc., Hillsdale, NJ. [Google Scholar]
- van Enkhuizen J, Geyer MA, Halberstadt AL, Zhuang X, Young JW, 2014. Dopamine depletion attenuates some behavioral abnormalities in a hyperdopaminergic mouse model of bipolar disorder. J Affect Disord 155, 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivometrics, 2002. The LifeShirt System™, Ventura, CA. [Google Scholar]
- Weafer J, de Wit H, 2013. Inattention, impulsive action, and subjective response to D-amphetamine. Drug Alcohol Depend 133 (1), 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YN, Wang L, Hartman L, Simcoe D, Chen Y, Laughton W, Eldon R, Markland C, Grebow P, 1998. Comparison of the single-dose pharmacokinetics and tolerability of modafinil and dextroamphetamine administered alone or in combination in healthy male volunteers. J Clin Pharmacol 38 (10), 971–978. [DOI] [PubMed] [Google Scholar]
- Young JW, Goey AK, Minassian A, Perry W, Paulus MP, Geyer MA, 2010. GBR 12909 administration as a mouse model of bipolar disorder mania: mimicking quantitative assessment of manic behavior. Psychopharmacology (Berl) 208 (3), 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, Kessler RM, 2008. Midbrain dopamine receptor availability is inversely associated with novelty-seeking traits in humans. J Neurosci 28 (53), 14372–14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.