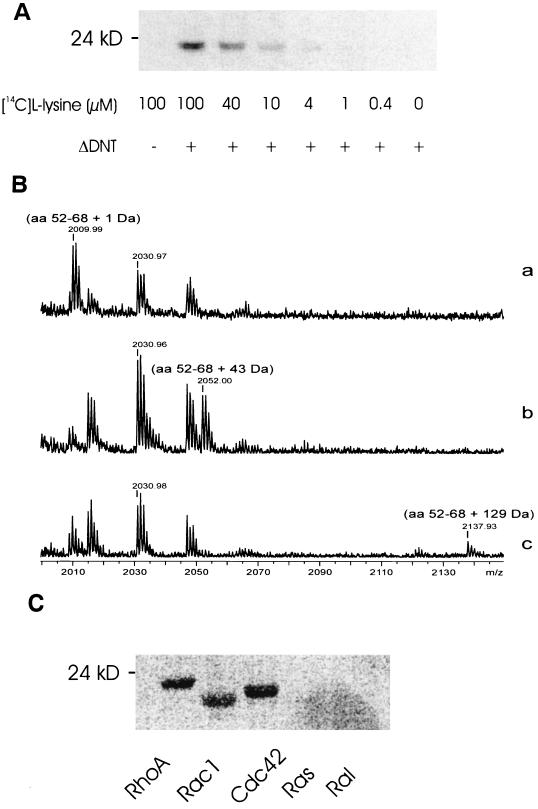
FIG. 1.
Cross-linking of small GTPases with l-lysine by DNT. (A) Recombinant RhoA (2 μM) was treated with ΔDNT (100 nM) at increasing concentrations of l-[14C]lysine. After incubation, the proteins were separated from free lysine by SDS-PAGE, and the modification was analyzed by phosphorimaging. (B) MALDI-MS spectra in gel digestion of modified RhoA. Gel plugs of RhoA modified in the absence (a) or presence (b) of ethylenediamine or in the presence of lysine (c), were excised and destained for 1 h in 40% acetonitrile–60% hydrogencarbonate (50 mM, pH 7.8). The plugs were subsequently dried in a vacuum centrifuge for 15 min. Thereafter, trypsin digestion was carried out for 12 h at 37°C. (a) Deamidation of glutamine-63 of RhoA by GST-ΔDNT results in a mass shift of the peptide of 1 Da. (b) Transglutamination of glutamine-63 of RhoA by GST-ΔDNT in the presence of ethylenediamine results in a mass shift of the peptide of 43 Da. (c) Transglutamination of glutamine-63 of RhoA by GST-ΔDNT in the presence of lysine results in a mass shift of 129 Da. (Note that the mass is given as mass + H+). (C) The small GTPases RhoA, Rac1, Cdc42, Ras, and Ral were incubated with GST-ΔDNT in the presence of 40 μM l-[14C]lysine. After incubation, the proteins were separated from free lysine by SDS-PAGE, and the modification was analyzed by phosphorimaging (shown).