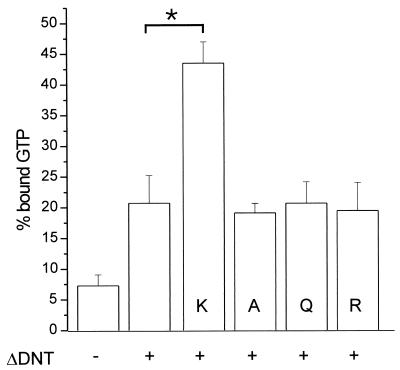
FIG. 2.
GTPase activity of modified RhoA. Recombinant Rho proteins were modified by GST-ΔDNT in the presence or absence of amino acids (K, l-lysine; A, alanine; Q, glutamic acid; R, arginine [20 mM each]). The reaction was stopped, and proteins were loaded with [γ-32P]GTP. Thereafter, the GTPase activity was stimulated by adding p50GAP. The hydrolysis of GTP was determined after 4 min by a filter binding assay (shown is the mean of the remaining bound radioactivity as a percentage of loaded radioactivity plus the SD of three independent experiments). Data with l-lysine are significantly different (∗, P < 0.001) from controls.