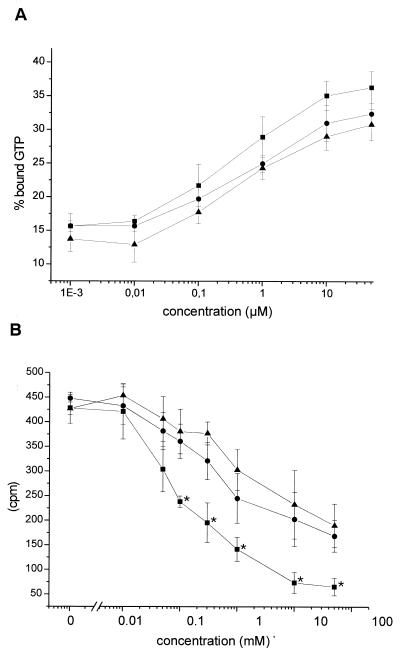
FIG. 6.
Substrate preference of ΔDNT. (A) GTPase activity. Small GTPases were incubated with GST-ΔDNT in the presence of increasing concentrations of lysine (■), putrescine (●), or spermidine (▴) as indicated. After incubation, the reaction was stopped, and the proteins were loaded with [γ-32P]GTP. GTPase activity was stimulated by adding p50GAP. The hydrolysis of GTP was determined by a filter binding assay (shown is the remaining bound radioactivity as a percentage of the loaded radioactivity as the mean ± the SD of three independent experiments). (B) Competition of l-lysine, putrescine, and spermidine with [14C]ethylenediamine as the substrate for DNT. Small GTPases were incubated with GST-ΔDNT in the presence of [14C]ethylenediamine and increasing concentrations of lysine (■), putrescine (●), or spermidine (▴) as indicated. Proteins were isolated from free ethylenediamine by precipitation with TCA and filtration. Incorporation of [14C]ethylenediamine was detected by counting (shown is the incorporated radioactivity as the mean ± the SD of three independent experiments). The data in the presence of l-lysine were significantly different (∗, P < 0.1) from those in the presence of putrescine (or spermidine).