Abstract
Background
Despite significant loss of bicarbonate during acute diarrhea, pediatric data are scarce with acute diarrhea/severe dehydration (ADSD) and severe non-anion-gap metabolic acidemia (sNAGMA). We planned to study their clinical profile, critical care needs, and outcome.
Patients
Children (1 month–12 years) with ADSD and sNAGMA (pH <7.2 and/or bicarbonate <15 mEq/L, and normal/mixed anion gap) admitted in Pediatric Emergency Department from January 2016 to December 2018 were enrolled. Children with pure high-anion-gap metabolic acidemia were excluded.
Methods
Medical records were reviewed retrospectively. The primary outcome was time taken to resolve acidemia. Secondary outcomes were acute care area free days in 5 days (ACAFD5), and adverse outcome as composite of Pediatric Intensive Care Unit (PICU) admission and/or death.
Results
Out of 929 diarrhea patients admitted for intravenous therapy, 121 (13%; median age, 4 months) had ADSD and sNAGMA. Median (IQR) pH was 7.11 (7.01–7.22); 21% patients had pH <7.00. Hyperchloremia (96%) and hypernatremia (45%) were common. About 12% patients each required inotropes and ventilation, while 58% had acute kidney injury (AKI). Median (IQR) time for resolution of acidemia among survivors was 24 (12, 24) hours. Thirty-two patients had adverse outcome. Higher grades of sNAGMA were associated with shock, AKI, coma, hypernatremia, hyperkalemia, adverse outcome, and lesser ACAFD5. Shock, ventilation, renal replacement therapy (RRT), and higher grades of sNAGMA were predictors of adverse outcome, with former two being independent predictors.
Conclusion
Severe non-anion-gap metabolic acidemia in children with ADSD is associated with organ dysfunctions, dyselectrolytemias, and lesser ACAFD5. Resolution of acidemia took unacceptably longer time. Higher grades of sNAGMA were a predictor of adverse outcomes. Trials are suggested to assess the role of additional bicarbonate therapy.
How to cite this article
Takia L, Baranwal AK, Gupta PK, Angurana SK, Jayashree M. Acute Diarrhea and Severe Dehydration in Children: Does Non-anion-gap Component of Severe Metabolic Acidemia Need More Attention? Indian J Crit Care Med 2022;26(12):1300–1307.
Keywords: Acute diarrhea, Alkali therapy, Bicarbonate, Metabolic acidemia, Non-anion-gap metabolic acidemia, Severe dehydration
Highlights
Severe non-anion-gap metabolic acidemia was seen in 13% of children with acute diarrhea and severe dehydration, and was associated with organ dysfunctions, dyselectrolytemias, and adverse outcome. Currently recommended World Health Organization (WHO) rehydration protocol delayed the resolution of metabolic acidemia until more than 24 hours. Additional intravenous bicarbonate was very rarely used.
Introduction
Diarrheal disease is the second leading cause of under-5 mortality,1,2 due to loss of water, bicarbonate, and other electrolytes.3–6 Bicarbonate precursors in the WHO oral rehydration solution and intravenous balanced solutions [e.g., Ringer lactate (RL), PlasmaLyte] recommended for deficit therapy serve to replenish bicarbonate losses.5–7 Widespread use of WHO protocol led to significant improvement in outcome of acute diarrhea–dehydration.5 Consequently, attention is getting shifted to critically ill patients with ADSD who have shock, life-threatening dyselectrolytemias, and severe metabolic acidemia.4,8,9
Despite realization of significant bicarbonate loss, published literature is predominantly focused on water loss and consequent dehydration, hypovolemic shock, and high-anion-gap metabolic acidemia (HAGMA), which respond to standard deficit therapy.5–7,10,11 In some patients, however, significant bicarbonate loss causes predominant non-anion-gap metabolic acidemia (NAGMA).11,12 Despite recommendations to use intravenous bicarbonate for severe acidemia due to bicarbonate loss,3,6,13,14 inordinate concerns of its adverse effects and absence of clinical evidence11 prevent its routine use. In this background, there is a felt need to characterize the clinical profile and management needs of patients with sNAGMA11,12 and to identify risk factors for continued adverse outcome in patients with ADSD.15 Thus, the current study was planned to delineate clinico-laboratory profile, critical care needs, time taken to resolution of acidemia and final outcome with currently recommended rehydration protocol, and predictors of adverse outcome among children with ADSD and sNAGMA. It may help answer if the non-anion gap component of metabolic acidemia in these children needs to be corrected as part of their rehydration therapy.
Patients and Methods
Study Setting, Eligibility Criteria, Definitions, and Classification
The study was conducted in 24-bed Pediatric Emergency Room (PER) and 15-bed PICU of a 1950-bed tertiary care teaching hospital. Children (age, 1 month to 12 years) with acute diarrhea admitted in PER for intravenous deficit therapy (IVDT) were screened, and those with severe dehydration and sNAGMA were included. Acute diarrhea and severe dehydration were defined and classified as per WHO criteria.5 Normal anion gap [Na+– (Cl–+HCO3–)] was defined as <16 mmol/L.16 Among patients with anion gap >16 mmol/L, ratio of delta anion gap and delta bicarbonate (lower limit of normal bicarbonate, 22 mmol/L) was obtained. Ratio <0.40 defined pure NAGMA, while ratio of 0.40–0.99 defined mixed metabolic acidemia.16 Severe non-anion-gap metabolic acidemia was defined as pH <7.20 and/or serum bicarbonate (SB) <15 mmol/L, PaCO2 <45 mm Hg, and anion gap <16 mEq/L.11,14 Among patients with anion gap >16 mmol/L, a delta ratio <1.0 guided the inclusion. Severe non-anion-gap metabolic acidemia was further classified as follows:
Grade I sNAGMA: Children with pH 7.11–7.20 and/or SB 10.1–15.0 mmol/L.
Grade II sNAGMA: Children with pH 7.01–7.10 and/or SB 5.1–10.0 mmol/L.
Grade III sNAGMA: Children with pH ≤7.00 and/or SB ≤5.0 mmol/L.
Children with pure HAGMA (anion gap >16 mmol/L and delta ratio >1.0), chronic/persistent diarrhea, renal tubular acidosis, chronic kidney or liver disease, elevated transaminases, diabetic ketoacidosis, poisoning, suspected/confirmed inborn error of metabolism, or receiving diuretics were excluded.
Financial Support, Ethical Clearance, and Patients’ Consent
It was a nonfunded dissertation project. The study protocol was approved by Institute Ethics Committee (INT/IEC/2019/000485 dated 15.03.2019). Being a retrospective chart review, written informed consent was waived off. The paper was approved by Departmental Review Board.
Study Design and Data Collection
For this retrospective study, data were collected from clinical records of patients, admitted in PER and PICU from January 2016 to December 2018. During the study period, 929 children with diarrhea were admitted for IVDT, out of which 121 (13.02%) were eligible. Demographic data, clinical status at admission, laboratory data, clinical management, and outcome were extracted in a predesigned pro forma.
Clinical Status at Admission and Management
The presence of fluid-refractory shock [persistence of hypotension (systolic blood pressure <5th centile for the age)] or need for vasoactive after >40 mL/kg of fluids in the first hour,17 respiratory failure requiring intubation and ventilation, AKI,18 and central nervous system (CNS) dysfunction at admission were recorded. Immediately after initial resuscitation in PER,17 IVDT was started with RL at a rate suggested in WHO protocol for children with different age, weight, and nutritional status.5 Patients in PER received basic critical care interventions, e.g., monitored fluid bolus, correction of deficit and dyselectrolytemias, oxygen therapy, inotropes, intubation, hand ventilation, clinical, and biochemical monitoring.19 Subsequently, patients were transferred to PICU if required. In PICU, patients were managed as per unit protocol for hemodynamic support, ventilation, peritoneal dialysis, sedation, nutritional, and nursing support.
Discharge Criteria
Discharge from PER was considered if (i) dehydration got corrected, (ii) metabolic acidemia got resolved, (iii) up to 3 stools in 24 hours, and (iv) normal oral intake was achieved. In some patients, decision to discharge was taken based on clinical status alone, without documenting resolution of acidemia. Patients are generally discharged from PER in two slots, in the morning and in the evening, due to administrative convenience and workload. For patients, in whom resolution of acidemia was not documented, time of discharge was taken as time of acidemia resolution. Patients transferred to PICU were shifted to pediatric wards after management and got discharged from there.
Outcome Variables
Primary outcome was time taken to resolve metabolic acidemia. Time to achieve target pH (>7.30) and target bicarbonate (>15 mmol/L) was recorded. Resolution of acidemia was defined by achieving pH >7.30 and/or bicarbonate >15 mmol/L. Secondary outcome variables were acute care area (ACA) stay and hospital stay among survivors, PICU stay among survivors of PICU admission, ACAFD5, mortality, and adverse outcome. We combined PER and PICU stay while calculating ACA stay and ACAFD5.20 Patients who died in PER or PICU were considered to have “zero” ACAFD5 as they were never free from ACAs. In our clinical setting, some patients leave hospital after getting counseled about futility of further care. These are categorized as Left Against Medical Advice (LAMA). We considered LAMA patients similar to death. Adverse outcome was a composite of transfer to PICU and/or in-hospital deaths/LAMA.
Statistical Analysis
Continuous variables between two and three groups were compared with Mann–Whitney and Kruskal–Wallis tests, respectively. Chi-square test (or Fisher exact test) was used for categorical variables. Point-biserial correlation was used to find correlation of pH and SB with presence of shock and AKI. Among survivors, time to achieve target pH and SB was described by Kaplan–Meier curves. Univariate analysis was done to identify predictors for adverse outcome. Independent predictors were identified by multivariable binomial logistic regression with forward selection procedure including predictors with p <0.05. A p-value of <0.05 indicated significance. All statistical analyses were done with SPSS software version 25 (IBM, New York, USA).
Results
Table 1 summarizes baseline characteristics of included patients. More than half of patients (n = 73, 60.3%) were <6 months of age, and two-thirds were underweight. Though boys dominated the cohort, girls had higher grades of sNAGMA (p = 0.002). Acute kidney injury, shock, and coma were present in 57.8%, 28.1%, and 8.2% of patients, respectively, at admission; increasing grade of sNAGMA was associated with significantly higher incidence (p-values; 0.004, 0.028, and 0.005, respectively). Among patients with shock, 41% (14/34) had fluid refractory shock. Eleven patients had respiratory failure requiring intubation and ventilation at admission, more so among grade III patients.
Table 1.
Baseline demographic and clinical characteristics of patients (n = 121)
| Characteristics | Total (n = 121) | Grade I (n = 50) | Grade II (n = 47) | Grade III (n = 24) | p-value |
|---|---|---|---|---|---|
| Age (months) [median (IQR)] | 4 (2, 8.5) | 3 (2, 7) | 4 (1.5, 8) | 5 (2, 9) | 0.55 |
| Age <6 months | 73 (60.3) | 31 (62) | 28 (59.5) | 15 (62.5) | 0.96 |
| Male: Female | 74:47 | 39:11 | 26:21 | 9:15 | 0.002 |
| Nutritional status | |||||
| Normal weight (WAZ >–2) | 40 (33.1) | 18 (36) | 15 (31.9) | 7 (29.2) | 0.97 |
| Moderate underweight (WAZ <–2 to >–3) | 27 (22.3) | 10 (20%) | 11 (23.4%) | 5 (20.8) | |
| Severe underweight (WAZ <–3) | 55 (45.5) | 22 (44) | 21 (44.7) | 12 (50) | |
| Duration of diarrhea [median (IQR)] | 3 (2, 5) | 2 (2, 3) | 3 (2, 5) | 3 (1, 4) | 0.20 |
| Fever | 74 (61.2) | 27 (54) | 33 (70.2) | 14 (58.3) | 0.25 |
| Vomiting | 95 (78) | 39 (78) | 37 (78.7) | 19 (79.2) | 0.99 |
| Shock | 34 (28.1) | 11 (18) | 11 (30.6) | 12 (50) | 0.028 |
| Respiratory failure | 15 (12.4) | 3 (6.0) | 7 (14.9) | 5 (20.8) | 0.15 |
| Acute kidney injury | 70 (57.8) | 22 (44) | 28 (59.6) | 20 (83.3) | 0.006 |
| Coma (GCS < 8) | 10 (8.3) | 1 (2) | 3 (6.4) | 6 (25) | 0.003 |
Values are in n (%) unless specified otherwise; WAZ, weight for age Z score; GCS, Glasgow coma scale; IQR, interquartile range
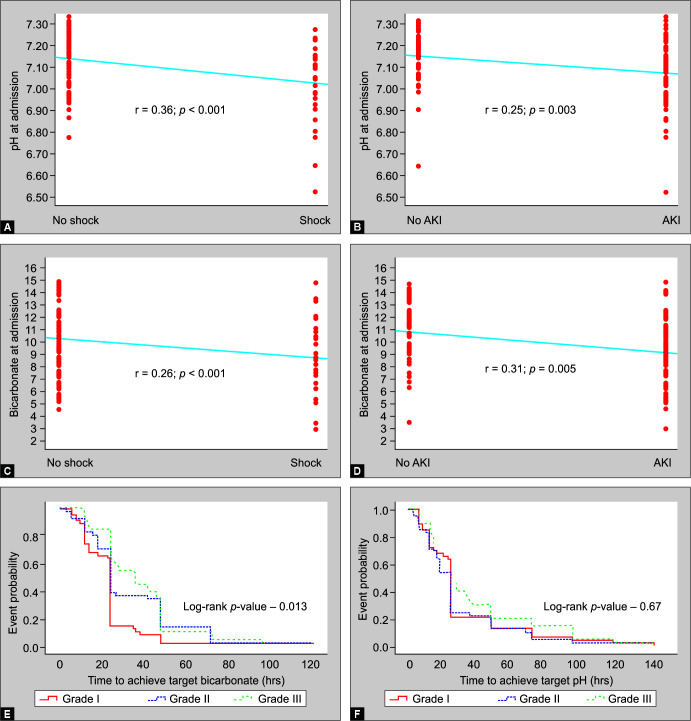
Table 2 shows baseline acid–base and electrolyte status. Median pH was 7.11 (range, 6.52–7.33), while median SB was 9.6 mmol/L (range, 2.9–14.8). A fifth of patients (n = 25) had pH <7.0, while more than half (n = 66) had SB <10 mmol/L. More than half of patients had hyperlactatemia, while a fifth had lactate >4 mmol/L. Interestingly, about 40% of patients in grade III had lactate >4 mmol/L compared with only 13% in grades I and II (p = 0.015). Lower pH and SB at admission were positively correlated with presence of shock (point-biserial correlation, r = 0.36 and 0.26; p <0.001 and 0.001, respectively) and AKI (point-biserial correlation, r = 0.25 and 0.31; p = 0.003 and 0.005, respectively) (Figs 1A to D). Hypernatremia was 4-fold more common than hyponatremia. Increasing grades of sNAGMA were associated with significantly higher incidence and severity of hypernatremia and hyperkalemia.
Table 2.
Baseline laboratory characteristics of patients (n = 121)
| Parameter | Total (n = 121) | Grade I (n = 50) | Grade II (n = 47) | Grade III (n = 24) | p-value |
|---|---|---|---|---|---|
| Hemoglobin (gm/dL) | 9.8 (8.12, 11.4) | 9.2 (7.9, 11.4) | 9.5 (8.5, 10.9) | 9 (8.8, 9.9) | 1.0 |
| Blood pH | 7.11 (7.01, 7.22) | 7.22 (7.18, 7.26) | 7.08 (7.03, 7.11) | 6.93 (6.88, 6.96) | <0.0001 |
| Blood pH, n (%) | |||||
| ≤7.0 | 25 (20.7%) | 0 (0) | 0 (0) | 24 (100%) | <0.0001 |
| >7.0–≤7.1 | 35 (28.9%) | 0 (0) | 36 (76.6%) | 0 (0) | |
| >7.1 | 61 (50.4%) | 50 (100%) | 11 (23.4%) | 0 (0) | |
| Serum bicarbonate (mmol/L) | 9.6 (7.6, 12) | 12.1 (11.1, 13.4) | 8.7 (7.5, 9.6) | 7.25 (5.5, 7.9) | <0.0001 |
| Serum bicarbonate (mmol/L) | |||||
| ≤5 | 4 (3.3%) | 0 (0) | 0 (0) | 4 (16.6) | <0.0001 |
| >5–≤10 | 62 (51.2%) | 1 (2%) | 41 (87.3) | 20 (83.3%) | |
| >10–15 | 55 (45.5%) | 49 (98%) | 6 (12.7%) | 0 (0) | |
| Anion gap | 11.15 (6, 14.8) | 12 (6, 14) | 11 (5, 14) | 11 (6, 20) | 0.6 |
| Anion gap >16 mmol/L, n (%) | 20 (16.5%) | 8 (16%) | 5 (10.6%) | 7 (33.3%) | 0.14 |
| Serum lactate (mmol/L) | 2.40 (1.8, 3.3) (n = 109) | 2 (2, 3) (n = 45) | 2 (2, 3) (n = 41) | 3 (2, 4) (n = 23) | 0.042 |
| Serum lactate (>2 mmol/L), n (%) | 67 (61.47%) (n = 109) | 25 (55.5%) (n = 45) | 25 (60.97%) (n = 41) | 17 (73.9%) (n = 23) | 0.34 |
| Serum sodium (mmol/L) | 143 (137, 152) | 141 (137, 149) | 147 (137, 154) | 145 (137, 158) | 0.27 |
| Hyponatremia (<135 mmol/L)$, n (%) | 14 (11.6%) | 7 (14%) | 5 (10.6) | 2 (8.3%) | 0.81 |
| Severe hyponatremia (<125, mmol/L), n (%) | 4 (3.3%) | 1 (2.0%) | 1 (2.1%) | 2 (8.3%) | 0.32 |
| Hypernatremia (>145 mmol/L)$, n (%) | 54 (44.6%) | 18 (36%) | 24 (51%) | 12 (50) | 0.0004 |
| Severe hypernatremia (>170 mmol/L), n (%) | 8 (6.6%) | 0 (0%) | 4 (8.4%) | 4 (16.6%) | 0.007 |
| Serum potassium (mEq/L) | 4.2 (3.6, 4.9) | 4.0 (3.5, 4.8) | 4.3 (3.7, 4.9) | 4.6 (3.9, 6.8) | 0.044 |
| Hypokalemia (<3.4 mmol/L)$, n (%) | 22 (18.2%) | 11 (22%) | 8 (16.8%) | 3 (12.5%) | 0.7 |
| Severe hypokalemia (<2.5 mmol/L), n (%) | 5 (4.1%) | 2 (4%) | 2 (4.2%) | 1 (4.2%) | 1 |
| Hyperkalemia (>5.4 mmol/L)$, n (%) | 19 (15.8%) | 3 (6%) | 8 (16.8%) | 8 (33.6%) | 0.006 |
| Severe hyperkalemia (>7.5 mmol/L), n (%) | 3 (2.5%) | 0 (0) | 0 (0) | 3 (12.6%) | 0.007 |
| Serum chloride (mmol/L) | 124 (117, 134) | 122 (116, 13) | 126 (120, 139) | 127 (120, 136) | 0.089 |
| Hypochloremia (≤96 mmol/L)$, n (%) | 1 (0.8%) | 0 (0%) | 0 (0%) | 1 (4.2%) | 0.19 |
| Hyperchloremia (≥108 mmol/L)$, n (%) | 116 (95.8%) | 48 (96%) | 45 (95.7%) | 23 (95.8%) | 1.0 |
Values are in median (IQR), unless specified otherwise,
$ Cut-offs for various dyselectrolytemias were defined as per Greenbaum et al.10
Figs 1A to F.
(A) Point-Biserial Correlation between pH at admission with presence of shock; (B) Point-biserial correlation between pH at admission with presence of acute kidney injury (AKI); (C) Point-biserial correlation between serum bicarbonate at admission with presence of shock; (D) Point-biserial correlation between serum bicarbonate at admission with presence of acute kidney injury (AKI); (E) Kaplan-Meier curves to compare time to achieve target pH (Log-rank test, p = 0.67) among survivors with different grades of severe non-anion gap metabolic acidemia; (F) Kaplan-Meier curves to compare time to achieve target serum bicarbonate (Log-rank test, p = 0.013) among survivors with different grades of severe non-anion-gap metabolic acidemia
Table 3 shows the critical care needs and outcomes of patients. Fourteen patients required inotropic support, 13 among these succumbed. Grade II and grade III patients required higher doses of inotropes (p = 0.06). Twelve patients (10%) developed new episodes of hypernatremia, while 25 patients (21%) developed hypokalemia during their clinical course. Seven (7/121, 5.8%) patients developed shock after initial resuscitation and deficit therapy. More patients in grade III (2/24, 8.3%) and grade II (3/47, 6.4%) developed fresh episode of shock than that in grade I (2/50, 4%). Despite severe metabolic acidemia, only 7 patients received rescue bicarbonate therapy, more among grade III patients (p = 0.035).
Table 3.
Critical care needs and outcomes among enrolled patients (n = 121)
| Sl. no. | Total (n = 121) | Grade I (n = 50) | Grade II (n = 47) | Grade III (n = 24) | p-value | |
|---|---|---|---|---|---|---|
| Critical care needs | ||||||
| 1 | Time taken for correction of dysnatremias (hours) | |||||
| Hypernatremia (n = 45) | 24 (24, 72) | 48 (6, 48) | 24 (24, 60) | 54 (24, 96) | 0.14 | |
| Hyponatremia (n = 9) | 48 (9, 48) | 24 (16, 48) | 30 (9, 48) | 42 (12, 72) | 0.21 | |
| 2 | Patients requiring inotropes | 14 (11.6%) | 3 (6%) | 7 (14.9%) | 4 (16.7%) | 0.268 |
| 3 | Maximum VIS score (n = 14) | 60 (60, 68.8) | 60 (60, 60) | 65 (60, 100) | 55 (40, 60) | 0.06 |
| 4 | Need for hand/Mechanical ventilation | 15 (12.3%) | 3 (5.8%) | 7 (14.9%) | 5 (20.8%) | 0.15 |
| 5 | Development of new episodes of dyselectrolytemias during management | 32 (26.4%) | 9 (18%) | 13 (27.7%) | 10 (41.7%) | 0.09 |
| • Hypernatremia | 12 (9.9%) | 5 (10.2%) | 4 (8.2%) | 3 (12.5) | 0.86 | |
| • Hyponatremia | 2 (1.7%) | 0 (0) | 1 (2.1%) | 1 (4.2%) | 0.45 | |
| • Hypokalemia | 25 (20.7%) | 6 (12.2%) | 13 (27.7%) | 6 (25%) | 0.15 | |
| • Hyperkalemia | 2 (1.7%) | 1 (2%) | 0 (0%) | 1 (4.2%) | 0.41 | |
| 6 | Rescue bicarbonate therapy | 7 (5.78%) | 1 (2%) | 2 (4.3%) | 4 (16.7%) | 0.035 |
| 7 | Renal replacement therapy | 4 (3.3%) | 1 (2%) | 2 (4.3%) | 1 (4.2%) | 0.681 |
| Primary outcome | ||||||
| 1 | Time to resolve acidemia (hours)a | 24 (12, 24) (n-108) | 24 (12, 24) (n-47) | 24 (12, 24) (n-41) | 24 (14, 48) (n-21) | 0.33 |
| 2 | Time to achieve target SB (hours)a | 24 (18, 42) (n-108) | 24 (13, 24) (n-47) | 24 (18, 48) (n-41) | 36 (24, 48) (n-21) | 0.006 |
| 3 | Time to achieve target pH (hours)a | 24 (12, 36) (n-108) | 24 (12, 24) (n-47) | 24 (12, 24) (n-41) | 24 (14, 48) (n-20) | 0.44 |
| Secondary outcomes | ||||||
| 1 | PICU admission | 22 (18.2%) | 5 (10%) | 8 (17%) | 9 (37.5%) | 0.016 |
| 2 | PICU-free days in 5 daysb | 2 (0, 2.5) (n = 22) | 2 (2, 2) (n = 5) | 2 (0, 2) (n = 8) | 2 (0, 2) (n = 9) | 0.291 |
| 3 | ACA-free days in 5 days | 2 (0, 2.25) | 3 (2, 4) | 2 (2, 3) | 1 (0, 3) | 0.006 |
| 4 | Death (including LAMA) | 13 (10.7%) | 3 (6%) | 6 (12.8%) | 4 (16.7%) | 0.33 |
| 5 | Adverse outcome (PICU admission and/or death/LAMA) | 32 (26.4%) | 8 (16%) | 13 (27.7%) | 11 (45.8%) | 0.024 |
Values are in median (IQR), unless specified otherwise,
aAmong survivors,
bAmong those shifted to PICU, VIS, vasoactive-inotropic score, PICU, Pediatric Intensive Care Unit, ACA, acute care area (Pediatric Emergency Room and Pediatric Intensive Care Unit), LAMA, left against medical advice
Of 121 patients, 108 patients survived. Target pH was documented in 76 survivors through blood gas, however, the same was not available in the remaining 32 survivors (29.6%). Target SB was achieved in 70 of the formers by the time the last blood gas was obtained. Among 13 nonsurvivors, target pH and SB could be achieved in two and four patients, respectively, before their demise. Survivors took a median duration of 24 h (IQR: 12 h, 24 h) for resolution of acidemia, with no difference between various grades of sNAGMA (p = 0.33). However, grade III patients took significantly longer time (median, 36 h) to achieve target SB compared to grade I and grade II patients (median, 24 h) (p = 0.006, Table 3; log-rank test p = 0.013, Fig. 1E). Grade II and grade III patients had significantly lesser ACAFD5 (p = 0.006), more transfers to PICU (p = 0.016), and adverse outcome (p = 0.024) (Table 3). Organ failures requiring life-support interventions (viz., shock, respiratory failure requiring ventilation, and AKI requiring RRT) were also significantly associated with adverse outcome (Table 4). Of them, presence of shock and need for ventilation at admission were identified as independent predictors of adverse outcome.
Table 4.
Univariate and multivariable analyses to identify predictors for adverse outcome (n = 121)
| Univariate analysis | |||||
|---|---|---|---|---|---|
| Sl. no. | Variables | Adverse outcome (n = 32) | No adverse outcome (n = 89) | Odds ratio | p-value |
| n (%) | n (%) | (95% CI) | |||
| 1 | Age <6 months | 19 (59.4) | 5 (65.2) | 0.78 (0.34–1.8) | 0.559 |
| 2 | Underweight (WAZ <–2 SD) | 20 (62.5) | 61 (68.5) | 0.765 (0.32–1.77) | 0.553 |
| 3 | Shock at admission | 17 (53.1) | 17 (19.1) | 4.8 (2–11.5) | <0.001 |
| 4 | Coma (GCS <8) | 5 (15.6) | 5 (5.6) | 3.11 (0.83–11.5) | 0.678 |
| 5 | Ventilation at admission | 14 (43.8) | 1 (1.1) | 68.44 (8.4–554.0) | <0.001 |
| 6 | Acute kidney injury | 22 (68.8) | 48 (53.9) | 1.87 (0.8–4.4) | 0.145 |
| 7 | Renal replacement therapy | 4 (12.5) | 0 (0) | 4.17 (3.0–5.7) | 0.004 |
| 8 | sNAGMA > Grade I | 24 (75) | 47 (52.8) | 2.11 (1.03–4.31) | 0.04 |
| 9 | Anemia (Hb ≤10 gm/dL) | 20 (64.5) | 38 (45.2) | 0.45 (0.19–1.06) | 0.067 |
| 10 | Hyponatremia (≤134 mmol/L) | 3 (9.3) | 11 (12.3) | 0.73 (0.19–2.8) | 0.75 |
| 11 | Hypernatremia (>146 mmol/L) | 15 (46.8) | 39 (43.8) | 1.06 (0.4–2.3) | 0.87 |
| 12 | Hypokalemia (≤3.4 mmol/L) | 6 (18.7) | 16 (17.9) | 1.05 (0.3–2.9) | 0.9 |
| 13 | Hyperkalemia (>5.5 mmol/L) | 5 (15.6) | 14 (15.7) | 0.95 (0.3–2.9) | 0.95 |
| 14 | Hyperlactatemia (>2 mmol/L) (n = 109) | 17 (58.6) (n = 29) | 50 (62.5) (n = 80) | 0.94 (0.47–1.88) | 0.71 |
| Multivariable analysis | |||||
| Variables | Regression co-efficient (SE) | Odds ratio | 95% CI | p-value | |
| 1 | Shock at admission | 0.538 | 4.25 | 1.49–12.13 | 0.007 |
| 2 | Ventilation at admission | 1.108 | 0.026 | 0.003–0.231 | 0.001 |
| 3 | sNAGMA > Grade I | 0.566 | 0.56 | 0.19–1.69 | 0.31 |
| 4 | Renal replacement therapy | 23076.91 | 0.00 | 0.00 | 0.99 |
WAZ, weight for age Z-score, GCS, Glasgow Coma Scale, sNAGMA, severe non-anion-gap metabolic acidemia
Discussion
Severe non-anion-gap metabolic acidemia was present in 13% of ADSD patients admitted in a tertiary care teaching hospital of a lower-middle income country. Patients were classified into three grades of increasing severity to better understand their clinical behavior, and to identify the most vulnerable group. Increasing grades of sNAGMA were associated with higher incidence and severity of shock and hyperlactatemia, CNS dysfunction (coma, respiratory failure), AKI, late resolution of acidemia, morbidity, and adverse outcome. Comparatively higher incidence of life-threatening hypernatremia and hyperkalemia was noticed. Late presentation to healthcare facilities, suboptimal acute care, poor implementation of time-tested and clinically proven resuscitative bundles, and late referral causing continued loss of fluid and bicarbonates may be responsible for the observed sickness.21,22 Few patients developed new issues, especially among those with higher grades. New hypokalemia episodes developed in 21% of patients; resolution of acidemia, diarrheal loss of potassium, and malnutrition might have been contributory.
Acute severe acidemia (pH <7.20) is shown to cause shock, respiratory failure, coma, seizures, and death.10–12,23 Though these effects are mostly described with HAGMA, the situation may not be different with NAGMA.11 The presence of sNAGMA in notable number of acute diarrhea patients is at variance to the common belief. Linear association of shock with lower pH and SB (Table 1, Figs 1A and C) suggests the former being a consequence of acidemia, rather than its cause in the studied sNAGMA patients. Similarly, respiratory failure, AKI, coma, and adverse outcome are likely to be consequences of sNAGMA. Universal hyperchloremia might have independently contributed to AKI and adverse outcome.11,13,23
Improved circulation with rehydration therapy in hypovolemic shock and hyperlactatemia would metabolize excess lactate to bicarbonate and would correct pure HAGMA faster. Renal regeneration and exogenous bicarbonate appear to play very little role in pure HAGMA.11 However, in patients with predominantly sNAGMA, rehydration alone may not be sufficient as circulating lactate would not provide sufficient bicarbonate. Such patients would need renal regeneration and additional alkali therapy.11 With the former being a slow process and likely to be impaired in AKI,3,6,11 resolution of acidemia (at a desired rate) is likely to require alkali therapy. World Health Organization rehydration protocol failed to resolve acidemia in about one-fifth of ADSD adults, who might predominantly have NAGMA.24 Being an independent predictor of mortality, the need of more than 24 hours to resolve acidemia is not acceptable.25
Additional slow infusion of bicarbonate may accelerate resolution of acidemia and hyperchloremia in this select group of patients,11 and may prevent further organ injuries. Its use as rescue therapy, that too only in seven patients, demonstrates a general reluctance due to potential adverse effects (e.g., hypokalemia, hypocalcemia, hypercapnia, paradoxical cerebrospinal fluid acidosis, hypernatremia, and hyperosmolarity). Adverse effects, however, should not preclude its use in situations where it is clearly indicated. Needless to say, close clinical and biochemical monitoring is essential. Though threshold pH and SB are debatable, a blood pH of 7.20 and SB of 15 mmol/L seems appropriate among patients with NAGMA.3,11
Despite severe acidemia, only 18% of patients could be transferred to PICU reflecting demand–supply gap for PICU beds. Considering the acuity of illness among patients who got transferred to PICU, the majority would have died if they would have landed in a secondary care hospital (e.g., District Hospital, Civil Hospital). Hence, transfer to PICU was included in the composite outcome measure of “adverse outcome”. Increasing grades of sNAGMA predicted adverse outcome on univariate analysis, however, it failed to be an independent predictor. This may be due to the strong association of increasing grades of sNAGMA, lower pH, and lower SB with incidence of shock at admission (Table 1, Figs 1A and C). Increasing grades of sNAGMA were also associated with severity of shock (e.g., need and dose of inotropes), and occurrence of new episodes of shock after initial rehydration therapy. With such overlap, shock seems to conceal the higher grades of sNAGMA as independent predictor.
To the best of our knowledge, this is the first attempt to study clinico-laboratory profile, critical care needs, and outcome of ADSD children with sNAGMA. We made an attempt to develop definitions of severe metabolic acidemia and its grading in children with predominant NAGMA from information and evidence available from the literature.11,14 It may seem to be liberal compared with definition of severe metabolic acidemia in children with predominant HAGMA. These definitions need to be validated further. In many patients, time of discharge from PER was taken as the timepoint of acidemia resolution without documenting the target pH/SB. Moreover, blood gases were obtained at discretion of the treating team, and not at regular intervals. Thus, the time of documented resolution of acidemia may not represent the earliest time when acidemia actually resolved. Both these factors are likely to overestimate the time taken to resolve acidemia. Despite these limitations, the sample size was large enough to derive clinically relevant conclusions. Trials to evaluate the addition of bicarbonate infusion to the prevalent rehydration protocols for ADSD patients with sNAGMA are suggested.
Conclusion
Severe non-anion-gap metabolic acidemia is common in children with ADSD in the studied setting. Higher grades of sNAGMA were associated with higher incidence and severity of organ dysfunctions, dyselectrolytemias, higher utilization of critical care interventions, and adverse outcome. Resolution of metabolic acidemia took more than 24 hours in the majority. Currently recommended rehydration protocol seems inadequate in resolving metabolic acidemia at a rate required to improve outcome in this select group of patients. Clinical trials to assess the role of additional slow bicarbonate infusion are suggested.
Authors’ Contributions
Lalit Takia (LT) enrolled patients, executed study protocol, did literature search, statistical analysis, and prepared the first draft of the paper. Arun Kumar Baranwal (AKB) conceptualized, planned and developed study design, supervised conduct of study and statistical analysis, prepared final draft of the paper, and will act as guarantor. Pramod Kumar Gupta (PKG) participated in development of study design, guided statistical analysis, and reviewed the final draft. Suresh Kumar Angurana (SKA) participated in development of study design. Muralidharan Jayashree (MJ) critically reviewed final draft of the paper.
Orcid
Lalit Takia https://orcid.org/0000-0002-3027-7006
Arun Kumar Baranwal https://orcid.org/0000-0003-4334-2652
Pramod Kumar Gupta https://orcid.org/0000-0002-6767-3520
Suresh Kumar Angurana https://orcid.org/0000-0001-6370-8258
Muralidharan Jayashree https://orcid.org/0000-0002-6149-1355
Footnotes
Source of support: Nil
Conflict of interest: None
References
- 1.Bhutta ZA, Das JK, Walker N, Rizvi A, Campbell H, Rudan I, et al. Interventions to address deaths from childhood pneumonia and diarrhoea equitably: What works and at what cost? Lancet. 2013;381(9875):1417–1429. doi: 10.1016/S0140-6736(13)60648-0. [DOI] [PubMed] [Google Scholar]
- 2.Troeger C, Forouzanfar M, Rao PC, Khalil I, Brown A, Reiner RC, et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(9):909–948. doi: 10.1016/S1473-3099. (17)30276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elliott EJ, Walker-Smith JA, Farthing MJ. The role of bicarbonate and base precursors in treatment of acute gastroenteritis. Arch Dis Child. 1987;62(1):91–95. doi: 10.1136/adc.62.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh M, Sankar J, Kumar A, Kumar UV, Lodha R, Kabra SK. Predictors of mortality in children admitted to the pediatric intensive care unit with acute gastroenteritis with severe dehydration. Indian J Pediatr. 2019;86(12):1142–1145. doi: 10.1007/s12098-019-03094-0. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization, Department of Child and Adolescent Health and Development. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers. Geneva: Department of Child and Adolescent Health and Development, World Health Organization; 2005. [Google Scholar]
- 6.Greenbaum LA. In: Nelson Essentials of Pediatrics. Kliegman RM, St. Geme JW, editors. Philadelphia: Elsevier; 2019. Deficit therapy. pp. 429–432. [Google Scholar]
- 7.Greenbaum LA. In: Nelson Essentials of Pediatrics. Kliegman RM, St. Geme JW, editors. Philadelphia: Elsevier; 2019. Maintenance and replacement therapy. pp. 425–428. [Google Scholar]
- 8.Naseem Md, Dubey AP, Mishra TK, Singh R. Effect of rehydration with normal saline versus ringer lactate on serum sodium level of children with acute diarrhea and severe dehydration: A randomized controlled trial. Indian Pediatr. 2020;57(6):519–522. 32562395 [PubMed] [Google Scholar]
- 9.Mahajan V, Saini SS, Sharma A, Kaur J. Ringer's lactate vs normal saline for children with acute diarrhea and severe dehydration: A double-blind randomized controlled trial. Indian Pediatr. 2012;49(12):963–968. doi: 10.1007/s13312-012-0251-x. [DOI] [PubMed] [Google Scholar]
- 10.Greenbaum LA. In: Nelson Essentials of Pediatrics. Kliegman RM, St. Geme JW, editors. Philadelphia: Elsevier; 2019. Electrolyte and acid-base disorders. pp. 389–424. [Google Scholar]
- 11.Kraut JA, Kurtz I. Treatment of acute non-anion gap metabolic acidosis. Clin Kidney J. 2015;8(1):93–99. doi: 10.1093/ckj/sfu126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kraut JA, Kurtz I. Use of base in the treatment of severe acidemic states. Am J Kidney Dis. 2001;38(4):703–727. doi: 10.1053/ajkd.2001.27688. [DOI] [PubMed] [Google Scholar]
- 13.Andrade OVB, Ihara FO, Troster EJ. Metabolic acidosis in childhood: why, when and how to treat. J Pediatr (Rio J) 2007;83(2 Suppl):S11–S21. doi: 10.2223/JPED.1616. [DOI] [PubMed] [Google Scholar]
- 14.Jaber S, Paugam C, Futier E, Lefrant J-Y, Lasocki S, Lescot T, et al. Sodium bicarbonate therapy for patients with severe metabolic acidaemia in the intensive care unit (BICAR-ICU): A multicentre, open-label, randomised controlled, phase 3 trial. Lancet. 2018;392(10141):31–40. doi: 10.1016/S0140-6736(18)31080-8. [DOI] [PubMed] [Google Scholar]
- 15.Zipursky A, Wazny K, Black R, Keenan W, Duggan C, Olness K, et al. Global action plan for childhood diarrhoea: Developing research priorities: Report from a Workshop of the Programme for Global Paediatric Research. J Glob Health. 2013;3(1):010406. doi: 10.7189/jogh.03.010406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraut JA, Madias NE. Serum anion gap: Its uses and limitations in clinical medicine. Clin J Am Soc Nephrol. 2007;2(1):162–174. doi: 10.2215/CJN.03020906. [DOI] [PubMed] [Google Scholar]
- 17.Davis AL, Carcillo JA, Aneja RK, Deymann AJ, Lin JC, Nguyen TC, et al. American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med. 2017;45(6):1061–1093. doi: 10.1097/CCM.0000000000002425. [DOI] [PubMed] [Google Scholar]
- 18.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 19.Ravikumar N, Baranwal AK. Fluid bolus in hypotensive septic shock: Need to encourage critical care interventions outside the formal PICU. Pediatr Crit Care Med. 2020;21(9):856–857. doi: 10.1097/PCC.0000000000002420. [DOI] [PubMed] [Google Scholar]
- 20.Ghosh S, Baranwal AK, Bhatia P, Nallasamy K. Suspecting hyperferritinemic sepsis in iron-deficient population: Do we need a lower plasma ferritin threshold? Pediatr Crit Care Med. 2018;19(7):e367–e373. doi: 10.1097/PCC.0000000000001584. [DOI] [PubMed] [Google Scholar]
- 21.Rudd KE, Kissoon N, Limmathurotsakul D, Bory S, Mutahunga B, Seymour CW, et al. The global burden of sepsis: Barriers and potential solutions. Crit Care. 2018;22(1):232. doi: 10.1186/s13054-018-2157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lakshminarayanan S, Jayalakshmy R. Diarrheal diseases among children in India: Current scenario and future perspectives. J Nat Sci Biol Med. 2015;6(1):24–28. doi: 10.4103/0976-9668.149073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Handy JM, Soni N. Physiological effects of hyperchloraemia and acidosis. Br J Anaesth. 2008;101(2):141–150. doi: 10.1093/bja/aen148. [DOI] [PubMed] [Google Scholar]
- 24.Soleimani A, Foroozanfard F, Tamadon MR. Evaluation of water and electrolytes disorders in severe acute diarrhea patients treated by WHO protocol in eight large hospitals in Tehran; a nephrology viewpoint. J Renal Inj Prev. 2016;6(2):109–112. doi: 10.15171/jrip.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jung B, Rimmele T, Le Goff C, Chanques G, Corne P, Jonquet O, et al. Severe metabolic or mixed acidemia on intensive care unit admission: Incidence, prognosis and administration of buffer therapy. A prospective, multiple-center study. Crit Care. 2011;15(5):R238. doi: 10.1186/cc10487. [DOI] [PMC free article] [PubMed] [Google Scholar]