Supplemental Digital Content is Available in the Text.
Key Words: Electroencephalography, qEEG, Neurophysiology, anti-NMDAR antibody encephalitis, Autoimmune encephalitis
Purpose:
Anti–N-methyl-d-aspartate receptor (anti-NMDAR) encephalitis is a form of autoimmune encephalitis associated with EEG abnormalities. In view of the potentially severe outcomes, there is a need to develop prognostic tools to inform clinical management. The authors explored whether quantitative EEG was able to predict outcomes in patients with suspected anti-NMDAR encephalitis.
Methods:
A retrospective, observational study was conducted of patients admitted to a tertiary clinical neuroscience center with suspected anti-NMDAR encephalitis. Peak power and peak frequency within delta (<4 Hz), theta (4–8 Hz), alpha (8 - 13 Hz), and beta (13–30 Hz) frequency bands were calculated for the first clinical EEG recording. Outcome was based on the modified Rankin Scale (mRS) score at 1 year after hospital discharge. Binomial logistic regression using backward elimination was performed with peak frequency and power, anti-NMDAR Encephalitis One-Year Functional Status score, age, and interval from symptom onset to EEG entered as predictors.
Results:
Twenty patients were included (mean age 48.6 years, 70% female), of which 7 (35%) had a poor clinical outcome (mRS 2–6) at 1 year. There was no association between reported EEG abnormalities and outcome. The final logistic regression model was significant (χ2(1) = 6.35, P < 0.012) with peak frequency in the delta range (<4 Hz) the only retained predictor. The model explained 38% of the variance (Nagelkerke R2) and correctly classified 85% of cases. Higher peak frequency in the delta range was significantly associated (P = 0.04) with an increased likelihood of poor outcome.
Conclusions:
In this exploratory study, it was found that quantitative EEG on routinely collected EEG recordings in patients with suspected anti-NMDAR encephalitis was feasible. A higher peak frequency within the delta range was associated with poorer clinical outcome and may indicate anti-NMDAR-mediated synaptic dysfunction. Quantitative EEG may have clinical utility in predicting outcomes in patients with suspected NMDAR antibody encephalitis, thereby serving as a useful adjunct to qualitative EEG assessment; however, given the small sample size, replication in a larger scale is indicated.
Anti–N-methyl-d-aspartate receptor (anti-NMDAR) encephalitis is the most common form of autoimmune encephalitis and is a severe but potentially reversible disorder resulting from immunoglobulin G (IgG) antibodies binding to the extracellular component of the NMDAR. It was initially described in young adult females with ovarian teratomas1; however, it is now recognized to affect males and females of all ages, mostly in the absence of tumors.2
The characteristic clinical phenotype of anti-NMDAR antibody encephalitis comprises initial nonspecific prodromal symptoms, followed by prominent neuropsychiatric features including psychosis, cognitive impairment, seizures, movement disorder, and autonomic instability.2–4 Later stages include impaired consciousness and hypoventilation, often necessitating intubation and intensive care.5 According to international consensus criteria, diagnosis is based on antibody detection, usually in the cerebrospinal fluid (CSF), in the presence of one or more common clinical features.6 Treatment of anti-NMDAR antibody encephalitis includes immunotherapy, typically consisting of steroids followed by plasmapheresis or pooled human immunoglobulin therapy, and when identified, tumor removal.2
Outcomes of anti-NMDAR encephalitis are highly variable. Approximately 75% of patients fully recover, or having mild sequelae, and the remaining 25% experience severe neurologic impairments or death.7 A range of clinical factors have been found to predict outcomes in this patient cohort, such as the time to start treatment.2 Recently, a five-point grading system termed, the “anti-NMDAR Encephalitis One-Year Functional Status” (NEOS) score, has been developed to aid prognostication.8
EEG in anti-NMDAR antibody encephalitis is abnormal in more than 80% of cases, with slow-wave abnormalities particularly common.9 Furthermore, in up to a third of patients, a highly specific wave form termed “extreme delta brush” has been reported, characterized by a rhythmic slow wave in the delta range with superimposed bursts of fast wave activity.10 Evidence suggests a temporal relationship between disease stage and EEG characteristics, starting with epileptiform discharges and followed by generalized slowing.5 EEG may also provide prognostic information.9,11,12 Quantitative EEG (qEEG) facilitates precise measurement of electrical brain activity13 and has been shown to differentiate anti-NMDAR antibody encephalitis from cryptogenic encephalitis14 and to predict the outcome in acute brain injury.15
In view of the potentially severe outcomes resulting from anti-NMDAR encephalitis, there is a need to develop objective prognostic tools to inform clinical management. We investigated whether qEEG was able to predict outcomes in patients with suspected anti-NMDAR encephalitis.
METHODS
Study Design
A retrospective observational study was conducted in patients with suspected anti-NMDAR encephalitis.6 The study was approved by the institutional review board of South London and Maudsley NHS Foundation Trust, part of Kings Health Partners. Inclusion required (1) the presence of one or more major symptom groups associated with anti-NMDAR encephalitis (abnormal psychiatric behavior or cognitive dysfunction, speech dysfunction, seizures, movement disorders, decreased consciousness, or autonomic dysfunction)6 and (2) antibodies to the GluN1 subunit of the NMDAR in serum or CSF. Patients were excluded if (1) they did not have an EEG performed during the episode or (2) an alternative diagnosis other than anti-NMDAR encephalitis was made during admission.
Patient Identification and Clinical Data Acquisition
A two-step process was involved in selecting cases. First, an electronic database of all patients tested for anti-NMDAR antibodies between July 2010 and May 2017 at Kings College Hospital (a tertiary clinical neuroscience center in London, United Kingdom) was reviewed to identify all patients who tested positive for anti-NMDAR antibodies in either serum or CSF. Second, the electronic hospital records of patients who tested positive for anti-NMDAR antibodies were scrutinized to confirm eligibility.
Sociodemographic investigation results and clinical information (including neuropsychiatric features, treatment, and outcome) were extracted. Patients were retrospectively assessed as to whether they met international consensus diagnostic criteria for probable or definite anti-NMDAR encephalitis; however, this was not used as part of the study eligibility because this diagnostic criteria was not introduced until the latter stage of the study inclusion period,6 and therefore, CSF immunoglobulin G (IgG) anti-GluN1 antibody testing (which forms a major component of the diagnostic criteria) was not routinely performed before then. The NEOS score was also calculated for each patient based on the clinical notes.
EEG Data Acquisition
In all cases, EEG was part of a routine diagnostic evaluation because of the presence of one or more neuropsychiatric features indicative of central nervous system disorder. The timing of EEG acquisition was determined by the treating clinician. The first clinical EEG recorded during the illness episode was designated the index recording. EEGs were recorded by a qualified technician using a multichannel Nicolet System One machine (CareFusion, San Diego, CA) using a sampling rate of 256 Hz. Recordings were collected from scalp electrodes placed according to the modified Maudsley electrode placement system. A 0.5 Hz low- and 70 Hz high-frequency filter was used for all recordings. The reference channel was Cz. EEGs were performed with the patient awake as confirmed by video telemetry. Epochs during periods of eye closure were selected to minimize ocular artefact.
Quantitative EEG Processing
Awake EEG was used for quantitative analysis. For each patient, six epochs (10 seconds duration) coinciding with eye closure were sampled. Each epoch was separated by 60 seconds unless there was visible baseline shift, muscular artefact, or epileptiform activity. If any of these features were present, the epoch was based on the nearest period thereafter that was free of these features. Spectral analysis was performed using fast Fourier transform. We selected channel C3–C4 for quantitative analysis based on evidence that this channel is able to detect neurophysiological signals specific to anti-NMDAR encephalitis.14
Peak power and peak frequency within delta (<4 Hz), theta (4–8 Hz), alpha (8-13 Hz), and beta (13–30 Hz) frequency bands were calculated for each epoch. The mean for each patient was then calculated before group comparisons. As we wanted to explore the feasibility of performing qEEG in routine clinical practice, we used the inbuilt software within the Nicolet System for qEEG processing.
Clinical Outcome
The primary outcome measure was modified Rankin Scale (mRS) score at 1 year after hospital discharge. The mRS is a 6-point scale of disability ranging between no symptoms (0) and death (6).16 A good outcome was defined a priori as a score of 0 to 1, corresponding with an absence of any significant disability. Outcomes were double-rated by two researchers based on clinical notes.
Statistical Analysis
Sociodemographic, clinical, and investigation findings (EEG, CSF analysis, and MRI) were summarized for the entire sample. Patients with a good versus a poor outcome were compared using an independent-samples t-tests for continuous data. We also calculated the standardized mean difference (Cohen's d) in peak frequency and power between the two groups. A χ2 or Fisher exact test was used, as appropriate, to compare categorical data.
Binomial logistic regression explored predictors of clinical outcome using a backward stepwise method. Peak frequency and peak power for the four frequency bands, NEOS score, age, and interval from symptom onset to index EEG were entered as predictors. Backward elimination was based on Wald statistic. All EEG processing and analyses were performed blind to clinical outcome. The significance level for all analyses was Data were analyzed using SPSS version 23.
RESULTS
Clinical Features of the Entire Sample
Eighty-two patients tested positive for anti-NMDAR antibodies during the study period, 20 of whom were eligible for inclusion. Fourteen patients (70%) were female and the median age was 48.6 years (range, 22–75 years). Three female patients (21%) were identified as having a teratoma. All 20 patients presented with a first episode of suspected anti-NMDAR antibody encephalitis and had anti-NMDAR antibodies detected in the serum. One patient underwent CSF testing for NMDAR antibodies, which was found to be positive, and met criteria for definite anti-NMDAR encephalitis.6 A further 12 patients met criteria for probable anti-NMDAR encephalitis, based on a rapid onset of symptoms and at least four distinct clinical indicators.6
Of the six major groups of symptoms associated with anti-NMDAR encephalitis,6 the modal value was four. The most common symptom group was “abnormal behavior or cognitive dysfunction” (n = 19; 95%). Within this group, the most common symptoms were confusion (n = 13; 65%), psychomotor agitation (n = 12; 60%), and psychosis (n = 11; 55%). The median interval from symptom onset to admission was 20 days (interquartile range, 7–62 days). One patient (5%) underwent admission to the intensive care unit during the course of admission.
The median interval from admission to index EEG was 4.5 days (interquartile range, 2–15 days). In all cases, the EEG was 20 to 30 minutes in duration. None of the EEGs analyzed were performed while a patient was mechanically ventilated. Of the sample, 14 patients (70%) had one or more EEG abnormalities. The most common were focal slowing (47%), generalized slowing (26%), and electrographic seizure activity (26%). Extreme delta brush was present in one case.
All patients had MRI brain scans, except one because of marked behavioral disturbance. Eleven patients (58%) had a clinically relevant MRI abnormality, with the most common being signal hyperintensity (n = 7; 37%).
Clinical Features by Outcome
At 1 year, 13 patients (65%) had a good clinical outcome (mRS 0–1) and 7 patients (35%) had a poor outcome (mRS 2–5). There were no significant associations between clinical outcome and demographic or clinical features. There was also no significant association between clinical outcome and reported EEG, MRI, or CSF abnormalities. Summary statistics are reported in Table 1.
TABLE 1.
Sample Demographic, Clinical, and Investigation Findings
| All Patients (N = 20) | Good Outcome (n = 13) | Poor Outcome (n = 7) | P | |
| Age (years) | 48.6 (SD 19.3) | 43.5 (SD 17.8) | 58.0 (SD 19.5) | 0.11 |
| Gender (female) | 14 (70%) | 9 (69%) | 5 (71%) | 1.00 |
| Ethnicity (white) | 12 (60%) | 9 (69%) | 3 (43%) | 0.36 |
| Admission (days) | 37.4 (SD 36.8) | 39.3 (SD 43.9) | 33.5 (SD 18.0) | 0.76 |
| Neuropsychiatric features | ||||
| Psychiatric or cognitive impairment | 20 (100%) | 13/13 (100%) | 7/7 (100%) | 1.00 |
| Speech dysfunction | 14 (70%) | 11/13 (85%) | 3/7 (43%) | 0.12 |
| Seizure | 10 (50%) | 6/13 (46%) | 4/7 (57%) | 1.00 |
| Movement disorder | 11 (55%) | 9/13 (69%) | 2/7 (29%) | 0.16 |
| Decreased consciousness | 10 (50%) | 6/13 (46%) | 4/7 (57%) | 1.00 |
| Autonomic dysfunction | 8 (40%) | 7/13 (54%) | 1/7 (14%) | 0.16 |
| MRI | ||||
| Clinically relevant abnormality | 11 (58%) | 9/13 (69%) | 2/6 (33%) | 0.16 |
| EEG | ||||
| Any abnormality | 14/20 (70%) | 8/13 (62%) | 6/7 (86%) | 0.35 |
| Electrographic slowing | 12/20 (60%) | 7/13 (54%) | 5/7 (71%) | 0.64 |
| Diffuse slowing | 5/20 (25%) | 3/13 (23%) | 2/7 (29%) | 1.00 |
| Focal slowing | 9/20 (45%) | 6/13 (46%) | 3/7 (43%) | 1.00 |
| Electrographic seizure | 5/20 (25%) | 5/13 (39%) | 1/7 (14%) | 0.35 |
| Extreme delta brush | 1/19 (5%) | 1/13 (8%) | 0/7 (0%) | 1.00 |
| Cerebrospinal fluid | ||||
| Pleocytosis | 4/15 (27%) | 4/9 (44%) | 0/6 (0%) | 0.10 |
| Oligoclonal bands | 3/15 (20%) | 3/9 (33%) | 0/6 (0%) | 0.26 |
Outcome based on modified Rankin scale (mRS) score at 1-year follow-up. Mean and SD reported for age and hospital admission. Frequency count and percentage reported for all other variables. Two-tailed independent samples t-tests were performed for continuous variables, and Fisher's exact tests was performed for categorical variables.
Quantitative EEG and Outcome
Mean peak power and frequency were calculated by outcome (Fig. 1). The standardized mean difference in peak frequency was greatest in the delta range (d = 1.25), with patients with a poor outcome having a higher peak frequency. The standardized mean difference in peak power was greatest in the theta range (d = 0.59), with patients with a poor outcome having a higher peak power (see Table 1, Supplemental Digital Content 1, http://links.lww.com/JCNP/A164).
FIG. 1.
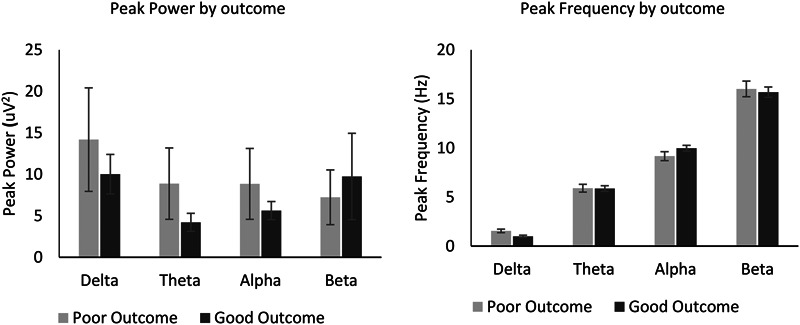
Quantitative EEG by outcome. Left panel: mean peak power across delta (<4 Hz), theta (4–8 Hz), alpha (8–13 Hz), and beta (13–30 Hz) frequency bands. Right panel: mean peak frequency across delta, theta, alpha, and beta frequency bands. Error bars represent 1 standard error.
Binomial logistic regression using backward elimination was performed with peak frequency and power, NEOS score, age, and interval from symptom onset to EEG entered as predictors. The final model was significant (χ2(1) = 6.35, p < 0.012), with peak frequency in the delta range (<4 Hz) the only retained predictor (see Table 2, Supplemental Digital Content 1, http://links.lww.com/JCNP/A164). The model explained 38% of the variance (Nagelkerke R2) and correctly classified 85% of cases. Higher peak frequency in the delta range was significantly associated (P = 0.04) with an increased likelihood of a poor clinical outcome (Fig. 2). A post hoc t-test found no association between peak frequency in the delta range and qualitative slow-wave abnormalities (P > 0.05).
FIG. 2.
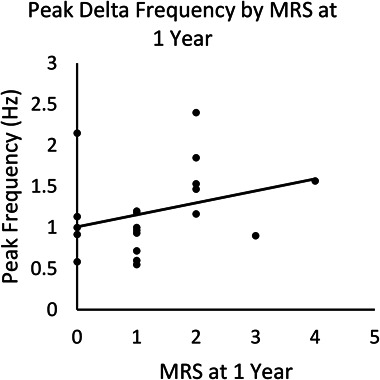
Scatter plot of peak frequency within delta range and modified Rankin Scale (mRS) at 1 year.
DISCUSSION
Summary of Main Findings
We explored qEEG as a prognostic tool in a sample of patients with suspected anti-NMDAR antibody encephalitis. We found that qEEG was predictive of later clinical outcome; in particular, patients with a higher peak frequency in the delta band were more likely to have a poor outcome. This finding suggests that peak frequency may be informative in predicting outcomes in this patient group. There was a moderate, though nonsignificant, difference in peak power within the slow frequency bands (delta and theta) between the two groups. The NEOS score was not found to predict outcomes at 1 year within our sample.
Overall, findings suggest that qEEG could be a potentially useful prognostic tool. Only one study to date has explored the prognostic role of qEEG parameters in anti-NMDAR antibody encephalitis.17 However, this is the first study to specifically evaluate the association between peak frequency and outcome. If replicated, qEEG could be used to inform future prognostic algorithms in anti-NMDAR antibody encephalitis.
Effects of NMDAR Antibodies on EEG
Systematic review evidence indicates that generalized slowing and other encephalopathic features are the most common EEG findings in anti-NMDAR encephalitis.9 The NMDAR is an ionotropic glutamate receptor, which is ubiquitously expressed throughout the brain. Experimental evidence suggests that the slowing of neuronal network oscillations observed in anti-NMDAR encephalitis are the result of antibodies disrupting NMDAR-mediated synaptic function.18 Furthermore, antibody-induced NMDAR internalization19,20 or altered surface distribution21 are hypothesized to underpin these changes.
The finding that patients with a higher peak frequency within the delta range went on to have poorer clinical outcomes was unexpected. Why this was the case not entirely clear? One possible explanation is that neural oscillations ordinarily occurring within the theta range were disrupted to the extent that they fell within the delta range. In view of these surprising results, replication is warranted. If this is established, exploration as to whether these findings are specific to anti-NMDAR encephalitis or are common to a wider range of disorders causing cerebral dysfunction would be indicated.
Although we did not find a statistically significant association between power and clinical outcome, a moderate effect size22 was observed in the alpha, theta, and delta range, with patients with a poor outcome having a higher peak power. The reason for this finding is unclear; however, it is plausible that increased power (especially in the lower frequency bands) reflects greater synchronized oscillatory activity. Further research with a larger sample, to facilitate greater statistical power, is recommended.
Contributions and Clinical Applications
This is the first study to our knowledge that has explored the potential role of peak frequency as a candidate prognostic marker in patients with suspected anti-NMDAR encephalitis. EEG is a widely available and well-tolerated investigative tool that is commonly used in patients with a suspected central nervous system disorder. Most studies of EEG in anti-NMDAR encephalitis have focused on its diagnostic applications.10 We found EEG abnormalities in 70% of patients, supporting the finding that EEG abnormalities are common.9 Furthermore, consistent with previous research, we identified electrographic slowing as the most common abnormality.2 However, in our sample, routine EEG assessment was not able to distinguish patients who had a good or poor clinical outcome. It is also notable that quantitative analysis was feasible in all patients included in the study. Together, this highlights the potential clinical utility of qEEG as an adjunct to standard qualitative interpretation23 in patients with suspected anti-NMDAR encephalitis. Our preliminary findings present a potential step toward a more objective measures of clinical prognostication using qEEG.
Strengths and Limitations
A strength of the study is that we used an existing clinical population with relatively few exclusion criteria, enhancing the generalizability of the findings. In addition, we used clinical software that is widely used in routine clinical practice to perform spectral analysis. There are, however, several limitations of the study that are important to acknowledge. These include a relatively small sample size, a retrospective design, and a reliance on serum antibody test results. This latter point reflects clinical practice during the period investigated, when CSF antibody testing was not routinely performed in the presence of a positive serum result. As a result, in most cases, it was not possible to confirm whether patients met international criteria for definite anti-NMDAR encephalitis.6 A further limitation of the study was the variability in time from onset of symptoms to EEG acquisition. We attempted to minimize this potentially important confounder by choosing the first EEG after hospital admission as the index EEG. Future research adopting a prospective design in a larger sample of patients with a diagnosis of definite anti-NMDAR encephalitis is recommended.
CONCLUSION
In this exploratory study, we demonstrated that it is feasible to perform qEEG on routinely collected EEGs in patients with suspected anti-NMDAR encephalitis. Higher peak frequency in the delta range was found to predict poor clinical outcome at one year. Findings suggest that qEEG has potential clinical utility as a prognostic tool in anti-NMDAR encephalitis; however, further research to replicate these findings is indicated.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. James Burge for his assistance with quantitative EEG analysis. Also, they thank the EEG technicians in the Department of Clinical Neurophysiology, King's College London for collecting the EEG recordings. Prof Anthony A. David was supported by a grant from the MRC [PCSBADC].
Footnotes
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.clinicalneurophys.com).
Contributor Information
Kieron Kumar, Email: kieron.kumar@slam.nhs.uk.
John G. Hanrahan, Email: johnghanrahan1@gmail.com.
Anthony Dalrymple, Email: anthony.hamilton.dalrymple@gmail.com.
Nandini Mullatti, Email: nandini.mullatti@nhs.net.
Nick Moran, Email: nickmoran@nhs.net.
Antonio Valentin, Email: antonio.valentin@kcl.ac.uk.
Lucy Gibson, Email: lucy.gibson@kcl.ac.uk.
Thomas A. Pollak, Email: thomas.pollak@kcl.ac.uk.
Anthony S. David, Email: anthony.s.david@ucl.ac.uk.
REFERENCES
- 1.Dalmau J, Tuzun E, Wu HY, et al. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007;61:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013;12:157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Diwani A, Handel A, Townsend L, et al. The psychopathology of NMDAR-antibody encephalitis in adults: a systematic review and phenotypic analysis of individual patient data. Lancet Psychiatry 2019;6:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson LL, Pollak TA, Blackman G, Thornton M, Moran N, David AS. The psychiatric phenotype of anti-NMDA receptor encephalitis. J Neuropsychiatry Clin Neurosci 2019;31:70–79. [DOI] [PubMed] [Google Scholar]
- 5.Irani SR, Bera K, Waters P, et al. N-methyl-D-aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non-paraneoplastic disorder of both sexes. Brain 2010;133:1655–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016;15:391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalmau J, Gleichman AJ, Hughes EG, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol 2008;7:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balu R, McCracken L, Lancaster E, Graus F, Dalmau J, Titulaer MJ. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology 2019;92:e244–e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillinder L, Warren N, Hartel G, Dionisio S, O'Gorman C. EEG findings in NMDA encephalitis: a systematic review. Seizure 2018;65:20–24. [DOI] [PubMed] [Google Scholar]
- 10.Schmitt SE, Pargeon K, Frechette ES, Hirsch LJ, Dalmau J, Friedman D. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology 2012;79:1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonderen AV, Arends S, Tavy DLJ, et al. Predictive value of electroencephalography in anti-NMDA receptor encephalitis. J Neurol Neurosurg Psychiatry 2018;89:1101–1106. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Liu G, Jiang MD, Li LP, Su YY. Analysis of electroencephalogram characteristics of anti-NMDA receptor encephalitis patients in China. Clin Neurophysiol 2017;128:1227–1233. [DOI] [PubMed] [Google Scholar]
- 13.Sarkis RA, Lee JW. Quantitative EEG in hospital encephalopathy: review and microstate analysis. J Clin Neurophysiol 2013;30:526–530. [DOI] [PubMed] [Google Scholar]
- 14.Foff EP, Taplinger D, Suski J, Lopes MB, Quigg M. EEG findings may serve as a potential biomarker for anti-NMDA receptor encephalitis. Clin EEG Neurosci 2017;48:48–53. [DOI] [PubMed] [Google Scholar]
- 15.Bagnato S, Boccagni C, Prestandrea C, Sant'Angelo A, Castiglione A, Galardi G. Prognostic value of standard EEG in traumatic and non-traumatic disorders of consciousness following coma. Clin Neurophysiol 2010;121:274–280. [DOI] [PubMed] [Google Scholar]
- 16.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607. [DOI] [PubMed] [Google Scholar]
- 17.Jiang N, Guan H, Lu Q, Ren H, Peng B. Features and prognostic value of quantitative electroencephalogram changes in critically ill and non-critically ill anti-NMDAR encephalitis patients: a pilot study. Front Neurol 2018;9:833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosch RE, Wright S, Cooray G, et al. NMDA-receptor antibodies alter cortical microcircuit dynamics. Proc Natl Acad Sci U S A 2018;115:E9916–E9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes EG, Peng X, Gleichman AJ, et al. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci 2010;30:5866–5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moscato EH, Peng X, Jain A, Parsons TD, Dalmau J, Balice-Gordon RJ. Acute mechanisms underlying antibody effects in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol 2014;76:108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mikasova L, De Rossi P, Bouchet D, et al. Disrupted surface cross-talk between NMDA and Ephrin-B2 receptors in anti-NMDA encephalitis. Brain 2012;135(pt 5):1606–1621. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum, 1988. [Google Scholar]
- 23.Nuwer M. Assessment of digital EEG, quantitative EEG, and EEG brain mapping: report of the American Academy of Neurology and the American Clinical Neurophysiology Society. Neurology 1997;49:277–292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


