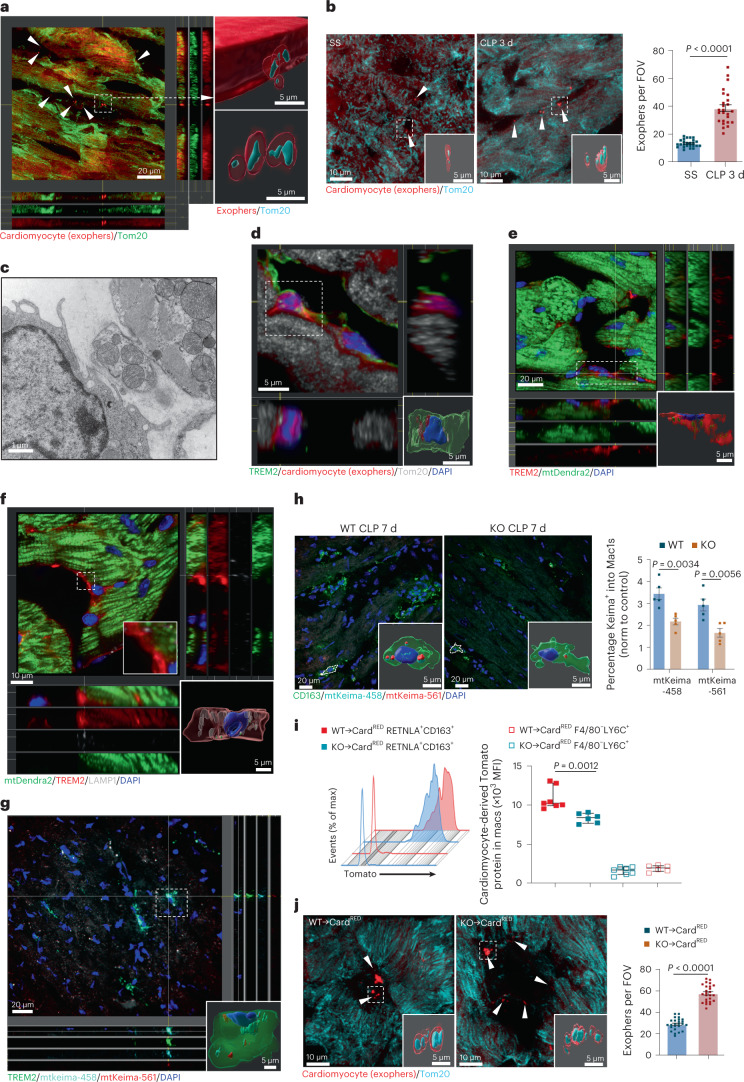
Fig. 5. TREM2 promotes the uptake of cardiomyocyte-derived mitochondria by Mac1 in septic heart.
a, Immunofluorescence images and 3D reconstruction showing presence of Tom20+ material in cardiomyocyte-derived exophers from hearts of septic CardRED mice. b, Presence of Tom20+ material in cardiomyocyte-derived exophers from hearts of CardRED mice at SS and 3 d after CLP. Graph showing exopher numbers per field of view (FOV); n = 6 per group. c, TEM image of a mononuclear cell taking up mitochondria by extended pseudopods in septic hearts (CLP 7 d). d, Images showing cardiomyocyte-derived exophers (red) containing mitochondria (Tom20, white) present in TREM2+ macrophages (green) from hearts of CardRED mice (n = 4). e, Cardiomyocyte-derived mitochondria (mt-Dendra2, green) present in TREM2+ macrophages (red) from hearts of MitoCard mice (n = 4). f, Cardiomyocyte-derived mitochondria (mt-Dendra2, green) localized in lysosomes (LAMP1, white) of TREM2+ macrophages (red) in hearts of MitoCard mice (n = 3). g, TREM2+ macrophages (green) took up cardiomyocyte-derived mitochondria (mt-Keima-458, cyan) and some mitochondria in an acidic environment (mt-Keima-561, red) from hearts of AAV9-Tnnt2-mt-Keima infected mice. h, CD163+ macrophages (green) engulfed cardiomyocyte-derived mitochondria in hearts of WT and Trem2−/− mice infected with AAV9-Tnnt2-mt-Keima, respectively. Graph showing percentages of mt-Keima+ mitochondria in CD163+ macrophages (n = 5 per group). For each animal, we randomly selected five visualization areas and five Mac1 cells were analyzed. The ratio of the area of mt-Keima mitochondria in a single Mac1 cell to the area of the same Mac1 cell was calculated. The ratio in the KO group was normalized to the ratio of the control group. i, The incorporation of cardiomyocyte-derived tdTomato protein in CD45+CD11b+F4/80+CD163+RETNLA+ macrophages and CD45+CD11b+F4/80−LY6C+ monocytes from WT → CardRED or KO → CardRED chimeras 7 d after CLP. Graph showing quantification of MFI of tdTomato (n = 6–7 per group). j, Presence of Tom20+ material (cyan) in cardiomyocyte-derived exophers (red) from hearts of WT → CardRED and KO → CardRED chimeras 7 d after CLP. Graph showing the exopher numbers per FOV (n = 6 per group). Scale bars are indicated in the images. Bars show as mean ± s.e.m. (b,h,j) and median with interquartile range (i). Two-sided P values were determined by unpaired t-test (b,h,j) and Mann–Whitney U-test (i). Results represent four (b), three (d–f) and two (g–j) independent experiments (Extended Data Figs. 6 and 7).