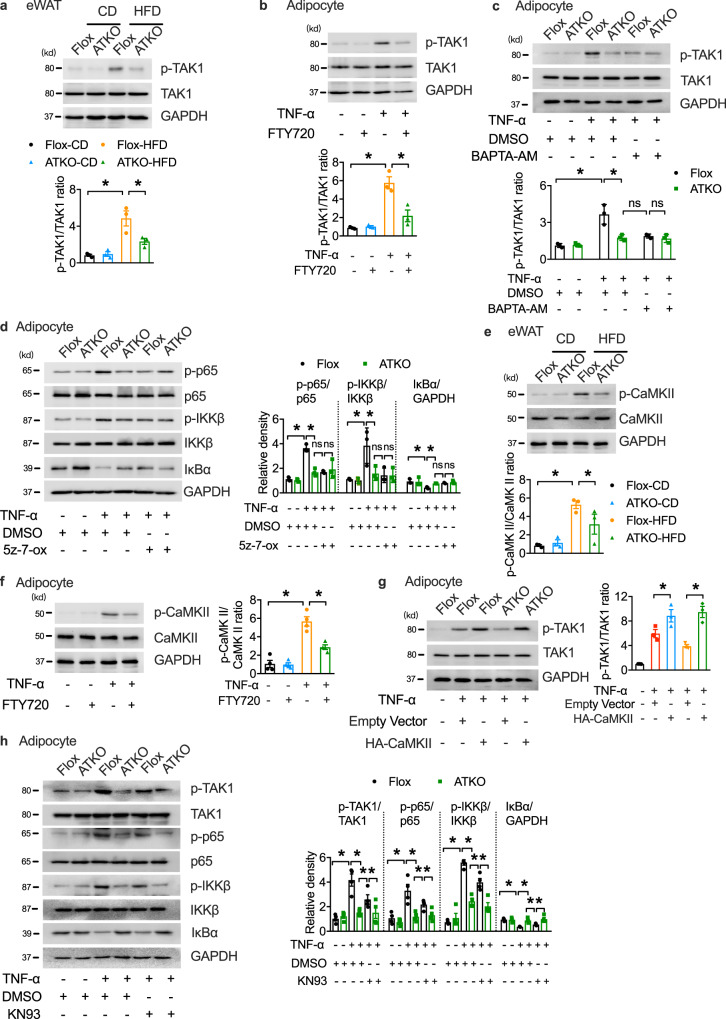
Fig. 5. TRPM7 promotes NF-κB signal by TAK1 activation partly dependent on CaMKII.
a Western blots of phosphorylation of TAK1 in eWAT from CD- and HFD- fed Flox and ATKO mice (n = 3 mice). b Western blot analysis of TAK1 phosphorylation in differentiated adipocytes pretreated with FTY720 (5 μM) for 15 min, and then treated with TNF-α (60 ng/ml) for 15 min as indicated (n = 3 biologically independent experiments). c Immunoblot analysis of TAK1 phosphorylation in cultured primary adipocytes treated with BAPTA-AM (10 μM; 30 min) followed by TNF-α administration (60 ng/ml; 15 min) (n = 3 biologically independent experiments). d Immunoblot analysis of adipocytes treated with TNF-α, DMSO or 5z-7-ox. Prior to TNF-α treatment (60 ng/ml; 15 min), adipocytes were pretreated with 5z-7-ox (100 nM) for 30 min (n = 3 biologically independent experiments). e Immunoblot analysis of p-CaMKII and total CaMKII in eWAT of indicated mice (n = 3 mice). f Phosphorylated and total CaMKII in FTY720 (5 μM; 15 min) loaded adipocytes in response to TNF-α (60 ng/ml; 15 min) were assessed by western blotting (n = 4 biologically independent experiments). g Western blot of phosphorylation of TAK1 in 3T3-L1 adipocytes transfected with HA-CaMKII or empty vector (n = 3 biologically independent experiments). h The levels of proteins in mouse cultured primary adipocytes treated with KN93 (10 μM; 30 min) or DMSO followed by TNF-α administration (60 ng/ml; 15 min) (n = 4 biologically independent experiments). All Statistical data in Fig. 5 were assessed using one-way ANOVA and are presented as mean ± SEM. *p < 0.05. Source data are provided as a Source Data file. kd, relative molecular weight in kilodalton.