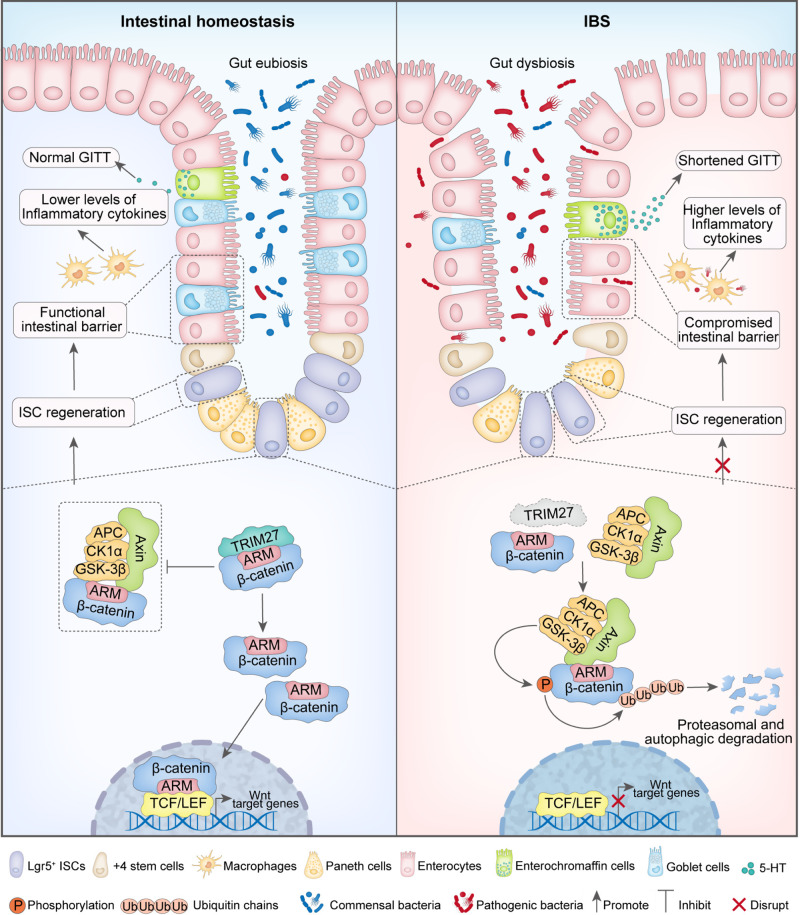
Fig. 9.
Proposed model showing the mechanism underlying TRIM27-mediated IBS symptoms. In the setting of intestinal homeostasis, TRIM27 competitively binds to the conserved Armadillo (ARM) repeat domain of β-catenin to disrupt the interaction between Axin and β-catenin, thus promoting the stabilization of β-catenin, followed by its transport from the cytoplasm to the nucleus to activate Wnt/β-catenin signaling pathway-mediated self-renewal of Lgr5+ ISC and the ensuing development and regeneration of the intestinal epithelium to maintain intestinal epithelial barrier integrity and gut homeostasis. In the absence of TRIM27, Axin directly interacts with the ARM domain of β-catenin to promote its phosphorylation and ubiquitination-mediated proteasomal and autophagic degradation, leading to suppression of the Wnt/β-catenin signaling pathway and defects in Lgr5+ ISC self-renewal. In turn, the defective self-renewal of Lgr5+ ISCs causes the disruption of intestinal epithelial barrier integrity and gut homeostasis, leading to the occurrence of IBS symptoms, including reduced goblet and Paneth cell numbers, elevated Tnf, Il1b and 5-hydroxytryptamine (5-HT) levels, a decreased gastrointestinal transit time (GITT) and dysregulation of the gut microbiota