Key Points
Question
Could paramedic risk stratification and point-of-care troponin testing result in cost savings for acute chest pain care at a population level?
Findings
In this economic evaluation of 188 551 patients attended by ambulance for chest pain in Victoria, Australia, the estimated annual statewide cost savings of prehospital risk stratification and troponin measurement was $6.45 million without using prehospital discharge and $42.84 to $71.84 million if prehospital discharge was used for low-risk patients.
Meaning
The findings suggest that prehospital risk assessment and point-of-care troponin testing may be viable based on cost savings alone, and investment should be considered by health services provided that safety is confirmed in prospective studies.
This economic evaluation uses cost-minimization analysis to estimate the cost savings of paramedic risk stratification of adult acute chest pain and point-of-care troponin testing.
Abstract
Importance
Prehospital point-of-care troponin testing and paramedic risk stratification might improve the efficiency of chest pain care pathways compared with existing processes with equivalent health outcomes, but the association with health care costs is unclear.
Objective
To analyze whether prehospital point-of-care troponin testing and paramedic risk stratification could result in cost savings compared with existing chest pain care pathways.
Design, Setting, and Participants
In this economic evaluation of adults with acute chest pain without ST-segment elevation, cost-minimization analysis was used to assess linked ambulance, emergency, and hospital attendance in the state of Victoria, Australia, between January 1, 2015, and June 30, 2019.
Interventions
Paramedic risk stratification and point-of-care troponin testing.
Main Outcomes and Measures
The outcome was estimated mean annualized statewide costs for acute chest pain. Between May 17 and June 25, 2022, decision tree models were developed to estimate costs under 3 pathways: (1) existing care, (2) paramedic risk stratification and point-of-care troponin testing without prehospital discharge, or (3) prehospital discharge and referral to a virtual emergency department (ED) for low-risk patients. Probabilities for the prehospital pathways were derived from a review of the literature. Multivariable probabilistic sensitivity analysis with 50 000 Monte Carlo iterations was used to estimate mean costs and cost differences among pathways.
Results
A total of 188 551 patients attended by ambulance for chest pain (mean [SD] age, 61.9 [18.3] years; 50.5% female; 49.5% male; Indigenous Australian, 2.0%) were included in the model. Estimated annualized infrastructure and staffing costs for the point-of-care troponin pathways, assuming a 5-year device life span, was $2.27 million for the pathway without prehospital discharge and $4.60 million for the pathway with prehospital discharge (incorporating virtual ED costs). In the decision tree model, total annual cost using prehospital point-of-care troponin and paramedic risk stratification was lower compared with existing care both without prehospital discharge (cost savings, $6.45 million; 95% uncertainty interval [UI], $0.59-$16.52 million; lower in 94.1% of iterations) and with prehospital discharge (cost savings, $42.84 million; 95% UI, $19.35-$72.26 million; lower in 100% of iterations).
Conclusions and Relevance
Prehospital point-of-care troponin and paramedic risk stratification for patients with acute chest pain could result in substantial cost savings. These findings should be considered by policy makers in decisions surrounding the potential utility of prehospital chest pain risk stratification and point-of-care troponin models provided that safety is confirmed in prospective studies.
Introduction
Acute chest pain accounts for 10% of ambulance attendances and is associated with significant health care costs and resource utilization.1,2,3,4 Chest pain is present in many serious diagnoses; therefore, existing guidelines recommend that most patients are transported to emergency departments (EDs) for further assessment, although 50% of patients are ultimately discharged without a specific diagnosis.3
Recently, studies have shown that paramedics can safely perform point-of-care troponin testing and risk stratification for patients with suspected acute coronary syndrome (ACS),5,6,7,8,9,10 which reduces ED length of stay.6,8 Although health outcome studies assessing this strategy are still emerging, prehospital risk stratification and point-of-care troponin testing may be noninferior to existing care processes with regard to the occurrence of early major adverse cardiac events.7,10 To date, most of these studies have been undertaken using conventional troponin assays, but point-of-care, high-sensitivity troponin (hsTn) assays have been developed and may see widespread availability in the near future. Nonetheless, there are significant infrastructure costs associated with equipping ambulances with point-of-care testing devices and hsTn cartridges in addition to paramedic training costs, and it is currently unclear whether a prehospital risk stratification and point-of-care troponin model would result in net cost savings.
We aimed, therefore, to perform a modeling study of the costs associated with prehospital point-of-care troponin pathways using a cost-minimization analysis based on linked ambulance, emergency, and hospital admission data for patients with acute chest pain. A cost-minimization analysis was selected because this intervention is focused on improving system efficiency, and emerging data have suggested that early outcomes are noninferior to existing care.7,10
Methods
This economic evaluation and data linkage study was approved by the Monash University human research ethics committee (approval number 11681). The committee deemed the study exempt from informed consent because it was low risk, and data were routinely collected and deidentified. The study followed the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) reporting guideline.11
The cost-minimization analysis compared the use of prehospital paramedic risk assessment and point-of-care troponin testing (with and without the use of prehospital discharge) with existing care processes using the state of Victoria, Australia, as the basis for the cost model. Victoria is a region of 6.7 million people residing in a 227 444-km2 area in the southeastern part of the country.4 Ambulance attendance data between January 1, 2015, and June 30, 2019, for consecutive adult patients with acute, nontraumatic chest pain without ST-segment elevation were linked to the Victorian Emergency Minimum Dataset and the Victorian Admitted Episodes Dataset to determine diagnoses and costs per episode of care. Full details regarding the cohort characteristics (sex, age, socioeconomic status, comorbidities, event location, and diagnoses) and linkage processes have been published previously4,12,13 and are included in the eMethods in Supplement 1. Indigenous Australian race was recorded in emergency or hospital data sets according to self-report, with this being a mandatory question for health services at time of patient admission. No other race and ethnicity data were collected in the included data sets.
Decision Model and Probabilities
We generated a decision tree model with 3 pathways to represent an existing chest pain care pathway (pathway 1) based on current chest pain management guidelines and prehospital risk stratification and point-of-care troponin pathways without (pathway 2) and with (pathway 3) the use of prehospital discharge (Figure 1).14 The existing care pathway included standard prehospital care (electrocardiogram, analgesia, intravenous cannulation) and transport of all patients with suspected ACS or those with suspicion of other serious illness to the nearest hospital. For patients with a history suggestive of ACS, a suspected ACS pathway would be undertaken, including hsTn protocols, with patients transferred to revascularization-capable centers if unavailable at the index hospital. Patients without ACS could be discharged following evaluation for other serious conditions, such as pulmonary emboli or aortic dissection, directly from the ED or following hospital admission.
Figure 1. Chest Pain Prehospital Risk Stratification and Point-of-Care Troponin Decision Tree.
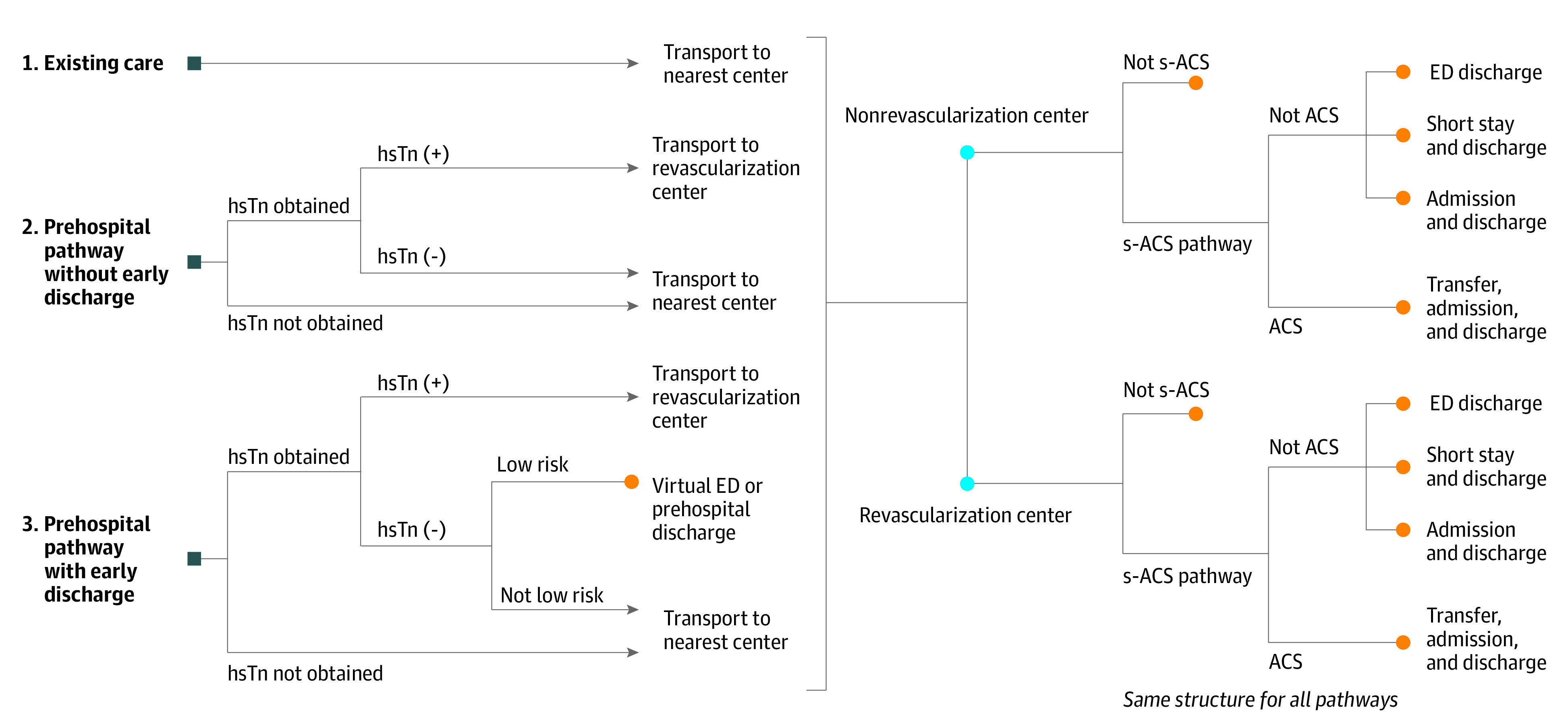
Square blue nodes indicate entry points to the model for each of the 3 pathways, including (1) existing care, (2) prehospital risk stratification and point-of-care troponin testing without prehospital discharge, and (3) prehospital risk stratification and point-of-care troponin testing with prehospital discharge using a virtual emergency department (ED). Orange circles indicate end points where costs are determined. On arrival to an ED, the same structure is followed for each pathway (right, with blue circles indicating receiving hospital center revascularization capabilities that patients are transported to), but probabilities are varied, including reduced admission rates for patients with suspected acute coronary syndrome (s-ACS) due to faster troponin results in EDs. hsTn indicates high-sensitivity troponin.
Under the prehospital risk assessment and point-of-care troponin pathways, costs could be altered in 3 ways: (1) patients with positive initial prehospital hsTn values could be transported directly to a revascularization center, avoiding the costs of nonrevascularization-capable index hospitals and transfers; (2) patients with suspected ACS without a final diagnosis of ACS could arrive at the hospital with the prehospital hsTn testing complete, allowing for earlier discharge from the ED and avoiding admission for some; and (3) patients classified as low risk by prehospital risk scoring could be referred to local medical services or a virtual ED service without transport to the hospital using single hsTn rule-out protocols, which are commonly used in EDs for very-low-risk patients (eTable 1 in Supplement 1).15,16,17 For patients being considered for nontransport to the hospital (pathway 3), a theoretical virtual ED would be proposed as a final safety net prior to prehospital discharge. Virtual EDs are a centrally operated emergency medicine telehealth service used by some centers in our region to reduce overcrowding. Specialist emergency physicians can remotely assess and manage low- and medium-acuity illness in patients in the community via video consultation.
For risk categorization in pathway 3, we assessed 2 risk scores, including the history, electrocardiogram, age, risk factors, and troponin (HEART) risk score, which has been validated for cohorts with suspected ACS in the prehospital setting,5,7,10 and the Early Chest Pain Admission Mortality and Myocardial Infarction (ECAMM) score, which is designed for undifferentiated chest pain presentations (rather than suspected ACS) but has not yet been prospectively validated.18 Risks of missing serious non-ACS diagnoses (eg, pulmonary embolism, heart failure, aortic dissection, pneumonia) are accounted for in a similar manner to standard ED pathways, with patients requiring classification as suspected ACS by paramedics and low-risk patients considered for prehospital discharge if clinical observations are normal (eMethods in Supplement 1), if pain has resolved, and after assessment by a virtual ED clinician. The ECAMM score also quantifies risk of admission for non-ACS diagnoses rather than ACS risk alone.
Branch probabilities were estimated based on a systematic review of the literature (eMethods in Supplement 1) and using the Ambulance Victoria–linked chest pain cohort (Table 1).8,18,19,20,21,22 Given that the Ambulance Victoria chest pain cohort represented an undifferentiated chest pain cohort, we estimated the probability of patients undergoing a suspected ACS assessment pathway by dividing the myocardial infarction rate in the Ambulance Victoria cohort by the myocardial infarction rate in the Rapid Assessment of Possible Acute Coronary Syndrome in the Emergency Department With High-Sensitivity Troponin T Study (RAPID-TnT) cohort, an Australian trial that enrolled patients planned to undergo a suspected ACS pathway.20 Probabilities of patients being classified as low risk by either the ECAMM or HEART scores were determined by calculating these scores using Ambulance Victoria comprehensive clinical data.18,23 Probabilities of patients avoiding admission due to a more rapid diagnosis were estimated based on studies assessing prehospital blood collection and trials comparing different hsTn algorithms (eMethods in Supplement 1),8,20,24 but to account for the uncertainty in this assumption, we used a more conservative range of 0% to 15%.
Table 1. Probabilities and Costs Based on the Literature and Linked Data Sources .
| Probability and costs | Value | Rangea | Source |
|---|---|---|---|
| Probability | |||
| Complete prehospital troponin and risk assessment | 0.792 | 0.50-0.90 | Stopyra et al,8 2021 |
| Patients with ACS with an initial prehospital positive troponin test result | 0.352 | 0.20-0.50 | Neumann et al,19 2016 |
| Patients with suspected ACS avoiding a short stay or hospital admission | 0.123 | 0.00-0.15 | Stopyra et al,8 2021; Chew et al,20 2019 |
| Patients with chest pain undergoing a suspected ACS pathwayb | 0.747 | 0.50-0.90 | Chew et al,20 2019; AV data set |
| Diagnosed with MI | 0.062 | 0.04-0.10 | AV data set |
| Nearest center not capable of revascularization | 0.323 | 0.15-0.50 | AV data set |
| Suspected ACS case classified as low risk by prehospital HEART score with resolution of pain and normal observations | 0.207 | 0.10-0.40 | Backus et al,21 2013; AV data set |
| Chest pain case classified as low or very-low risk by prehospital ECAMM score with normal troponin level and resolution of pain | 0.412 | 0.20-0.50 | Dawson et al,18 2022; AV data set |
| Annual proportion of point-of-care devices requiring repairs or replacement following warranty expirationc | 0.050 | 0.00-0.10 | Author consensus |
| Estimated costs (Australian $) | |||
| Attendance outcome and disposition | |||
| Nonrevascularization center | |||
| ED discharge | 2448 | Fixed | AV data set |
| Short stay discharge | 4145 | Fixed | AV data set |
| Admission (non-ACS) | 10 098 | Fixed | AV data set |
| Transfer (ACS) | 24 195 | Fixed | AV data set |
| Not suspected ACS | 5519 | Fixed | AV data set |
| Revascularization center | |||
| ED discharge | 2275 | Fixed | AV data set |
| Short stay discharge | 4069 | Fixed | AV data set |
| Admission (non-ACS) | 11 292 | Fixed | AV data set |
| Admission (ACS) | 16 512 | Fixed | AV data set |
| Not suspected ACS | 5974 | Fixed | AV data set |
| Low-risk classification and not transported to the hospital | 685 | Fixed | AV data set |
| Point-of-care troponin device costs | |||
| iStat analyzers (annual)d | 972 000 | Fixed | Abbott Point of Care |
| iStat simulators (annual)d | 21 500 | Fixed | Abbott Point of Care |
| hsTn cartridge (cost per attendance)e | 16 | Fixed | Abbott Point of Care |
| Paramedic education costs (annual)f | 502 380 | Fixed | Australian Government |
| Virtual ED costs | |||
| 24/7 ED consultant physician (annual)g | 1 921 303 | Fixed | AMA Victoria |
| 24/7 Administration staff (annual)h | 311 784 | Fixed | Victoria State Government |
| Infrastructure and operational (annual) | 100 000 | Fixed | Author consensus |
Abbreviations: ACS, acute coronary syndrome; AV, Ambulance Victoria; ECAMM, Early Chest Pain Admission Mortality and Myocardial Infarction; ED, emergency department; HEART, history, electrocardiogram, age, risk factors, and troponin; hsTn, high-sensitivity troponin; MI, myocardial infarction.
Range indicates limits of β-distribution for the probabilities used in the multivariable probabilistic sensitivity analyses.
Rate of MI in the AV data set of undifferentiated chest pain (6.2% MI) divided by the rate of MI in the Rapid Assessment of Possible Acute Coronary Syndrome in the Emergency Department With High-Sensitivity Troponin T Study cohort with suspected ACS (8.3% rule-in MI).
Annual proportion of point-of-care devices requiring repairs or replacement following a 2-year warranty calculated by multiplying the probability value by total annual cost of fleet analyzers and simulators.
iStat analyzer and simulator costs assume a 5-year expected device lifetime for a statewide ambulance fleet of 810 response teams with 215 branches requiring 1 analyzer per team and 1 simulator per branch.
High-sensitivity troponin cartridge cost per attendance accounts for an estimated 10% of cartridges being discarded due to storage issues, mishandling, device errors, or other unforeseen events.
Paramedic education costs estimated at an additional 3 hours annually for 6000 staff at a rate of $27.91/h (midpoint of the advanced life support paramedic pay scale), with additional education occurring during regular teaching periods (40 hours per year).
Virtual ED consultant physician annual cost estimated based on hourly rates for a year 3 consultant ED physician staffing a single statewide virtual ED 24 hours per day 7 days per week (accounting for out-of-hours shift penalties).
Annual cost estimated based on weekly rates and out-of-hours allowances for public hospital administration workers (grade 1, level 3).
Cost Estimates
Costs were summed for each pathway in the decision tree and reflect Australian dollars ($) in the 2020-2021 financial year. Ambulance costs were determined from 2021-2022 Victorian estimates of cost per transport type. Full details regarding cost calculations, including the case-mix funding models used in Victoria, are provided in the eMethods and eTables 2 and 3 in Supplement 1.
Point-of-care troponin device costs were provided by Abbott Point of Care Inc, with fleet setup costs for Victoria determined based on requiring 1 iStat analyzer for each of 810 ambulance response teams and 1 simulator for each of 215 ambulance branches. To account for the need for device repairs or replacements following a 2-year warranty period, we included a probability of device replacement estimated over a range of 0% to 10% per year for years 3 to 5 of an estimated 5-year device life span.
Paramedic training in our region is completed during preallocated teaching periods (40 hours per year); however, we accounted for an additional 3 hours of education for device use and patient selection per year. For the virtual ED assessment (pathway 3), costs were estimated assuming a theoretical centralized virtual ED staffed by a consultant emergency physician and administration staff 24 hours per day, 7 days per week. Costs per attendance (eg, hsTn cartridges) assumed an annual statewide ambulance caseload of 60 000 patients with chest pain without ST elevation over the first 5 years of operation,4 with the additional assumption that approximately 10% of cartridges might be discarded due to storage issues, mishandling, device errors, or other unforeseen events.
Statistical and Sensitivity Analysis
The primary analysis involved multivariable probabilistic sensitivity analyses used to estimate mean costs and cost differences among pathways. For probabilities, β-distributions were used with parameters based on previous literature (Table 1). A Monte Carlo simulation was performed with 50 000 iterations to determine means and 95% uncertainty intervals (UIs) for costs per ambulance attendance and statewide annual costs. Analyses were performed between May 17 and June 25, 2022, using Excel, version 1808 (Microsoft Corporation) software with the Ersatz add-in for Monte Carlo simulations.
Several 1-way sensitivity analyses were performed by varying individual input probabilities over clinically plausible ranges to assess their association with model results, and thresholds were calculated if present. Sensitivity analyses were performed on 6 variables, varying the proportion of patients (1) for whom the prehospital risk assessment and point-of-care hsTn assay are complete; (2) who have a positive initial prehospital troponin level; (3) who avoid a short stay or hospital admission (for suspected ACS not diagnosed as ACS); (4) who undergo a suspected ACS pathway; (5) who are transported initially to a nonrevascularization-capable center; and (6) who are classified as low risk, allowing prehospital discharge (among those with suspected ACS). Three additional multivariable probabilistic sensitivity analyses were performed using the primary analysis but varying (1) the inpatient cost inputs to the model over a range of 10% to 30%; (2) the costs of the ambulance attendance over a range of 10% to 100% for patients classified as low risk, reviewed by the virtual ED, and not transported to the hospital in the prehospital pathway that incorporated prehospital discharge; and (3) the costs for patients who might be transported to revascularization centers unnecessarily if hsTn results are elevated in the absence of ACS, assuming a 0% to 10% false-positive rate range and an increase in total ambulance and hospital costs among these patients ranging from 20% to 50%. Break-even points and cumulative costs across the first 5 years of operation in Victoria for each pathway were estimated by including the costs of equipping the ambulance fleet with the point-of-care device at the commencement of the 5 years rather than as an annualized cost.
Results
In total, 188 551 patients with acute chest pain without ST-segment elevation and with linked emergency and admission data were transported by Ambulance Victoria to public hospitals during the study period (eFigure 1 in Supplement 1), which represented the entry point to the decision tree. Mean (SD) age was 61.9 (18.3) years, 50.5% were female (49.5% male), and 2.0% were Indigenous Australian. The percentage of patients diagnosed with ACS was 9.6%, and 32.3% were initially transported to nonrevascularization centers. Further details regarding cohort characteristics and diagnoses in addition to attendance outcome and disposition within the Ambulance Victoria–linked chest pain cohort are presented in eTables 4 and 5 in Supplement 1.
Initial setup costs to equip the ambulance fleet with point-of-care troponin capabilities were estimated at $4.97 million (iStat devices, $4.86 million; simulator devices, $0.11 million), equating to an annual expenditure of $0.99 million assuming a device life span of 5 years. For the pathway without prehospital discharge, annual costs, including setup ($4.97 million), hsTn cartridges ($0.74 million), paramedic education ($0.50 million), and device replacements ($0.05 million), were estimated at $2.27 million. For the pathway with prehospital discharge, annual virtual ED costs were estimated at $2.33 million, equating to a total annual cost of $4.60 million.
Primary Analysis
Mean cost per attendance and mean annual costs for each pathway in the primary multivariable probabilistic sensitivity analysis are shown in Table 2. Annual cost savings for the prehospital point-of-care troponin pathway without prehospital discharge was $6.45 million (95% UI, $0.59-$16.52 million), with prehospital discharge using the HEART risk score saving $42.84 million (95% UI, $19.35-$72.26 million) and prehospital discharge using the ECAMM score saving $71.84 million (95% UI, $36.54-$116.14 million). From the 50 000 Monte Carlo iterations, the prehospital troponin model was cost minimizing in 94.1% of iterations without prehospital discharge and 100% of iterations with prehospital discharge (both HEART and ECAMM scores).
Table 2. Estimated Costs and Savings Using a Prehospital Point-of-Care Troponin Testing and Paramedic Risk Stratification Pathway Compared With Existing Care.
| Scenario | Cost per attendance, Australian $ | Total annual cost, millions of Australian $a | ||
|---|---|---|---|---|
| Mean (95% UI)b | Difference (95% UI)b | Mean (95% UI)b | Difference (95% UI)b | |
| Existing care pathway | 6773 (6722 to 6826) | NA | 406.37 (403.30 to 409.53) | NA |
| Prehospital hsTn pathway | ||||
| Without prehospital discharge | 6665 (6485 to 6812) | −107 (−275 to 10) | 399.92 (389.11 to 408.75) | −6.45 (−16.52 to 0.59) |
| With prehospital discharge (HEART) | 6059 (5564 to 6456) | −714 (−1204 to −323) | 363.53 (333.81 to 387.37) | −42.84 (−72.26 to −19.35) |
| With prehospital discharge (ECAMM) | 5575 (4837 to 6164) | −1197 (−1936 to −609) | 334.52 (290.22 to 369.83) | −71.84 (−116.14 to −36.54) |
Abbreviations: ECAMM, Early Chest Pain Admission Mortality and Myocardial Infarction; HEART, history, electrocardiogram, age, risk factors, and troponin; hsTn, high-sensitivity troponin; NA, not applicable; UI, uncertainty interval.
Based on 60 000 ambulance attendances for chest pain without ST-segment elevation per year in Victoria, Australia.
The 95% UIs are based on output of probabilistic sensitivity analysis using Monte Carlo simulation with 50 000 iterations.
Sensitivity Analyses
Results were robust in univariable sensitivity analysis with variation of 6 input parameters across their respective probability ranges (Figure 2; eFigure 2 in Supplement 1). The hsTn pathway without prehospital discharge was no longer cost saving if the percentage of patients with suspected ACS avoiding a short stay or hospital admission was less than 0.2%. No thresholds were identified for any of the other input parameters, with pathways 2 and 3 remaining cost minimizing in all other scenarios. In each of the further multivariable probabilistic sensitivity analyses, results were comparable to the primary analysis, accounting for higher inpatient costs, higher ambulance costs, and false-positive hsTn tests (eTables 6-8 in Supplement 1).
Figure 2. Univariable Sensitivity Analyses of Prehospital Risk Stratification and Point-of-Care Troponin Assay Costs.
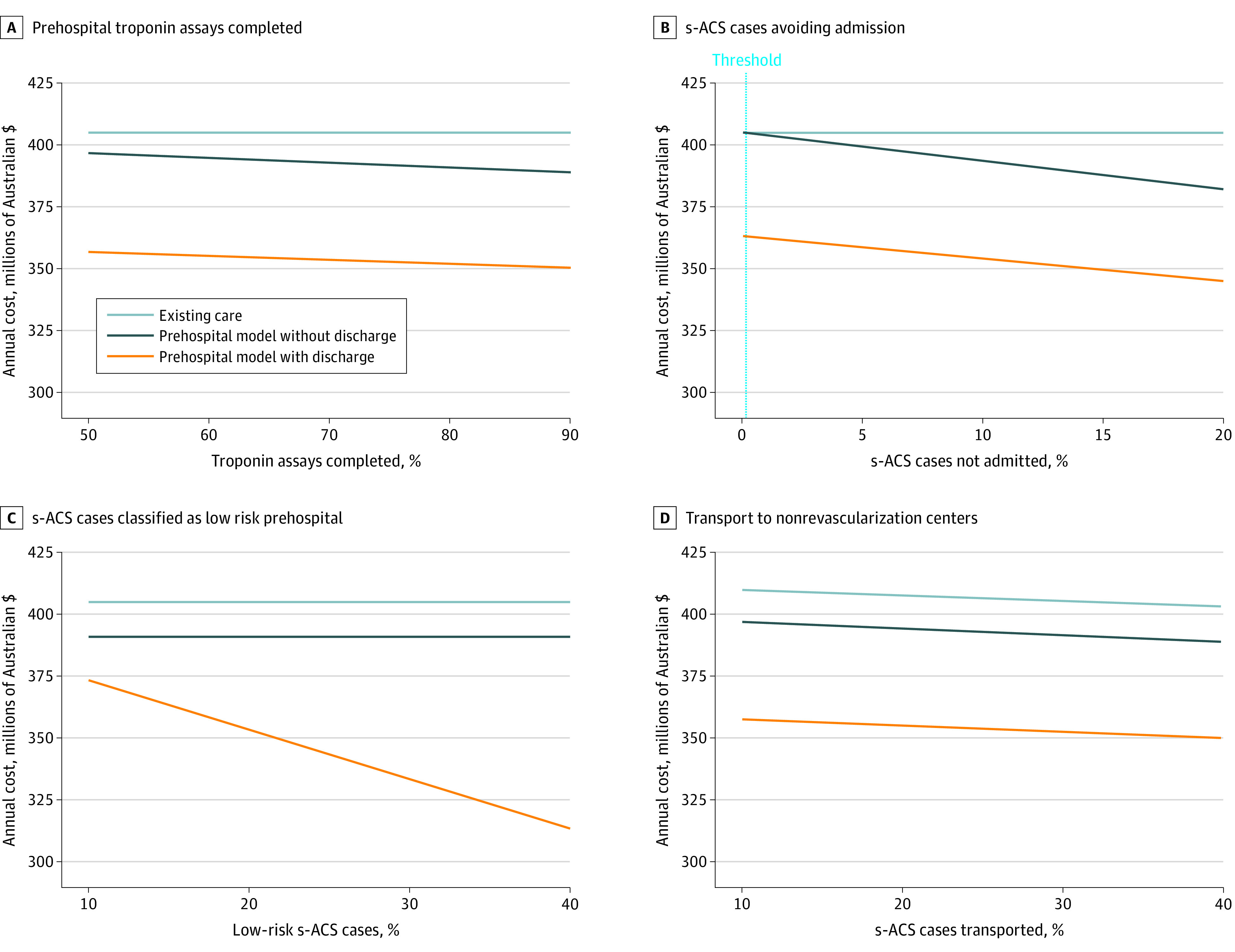
The prehospital model without discharge was no longer cost saving if the percentage of cases of suspected acute coronary syndrome (s-ACS) avoiding an admission was less than 0.2%.
Cumulative Costs Over Time
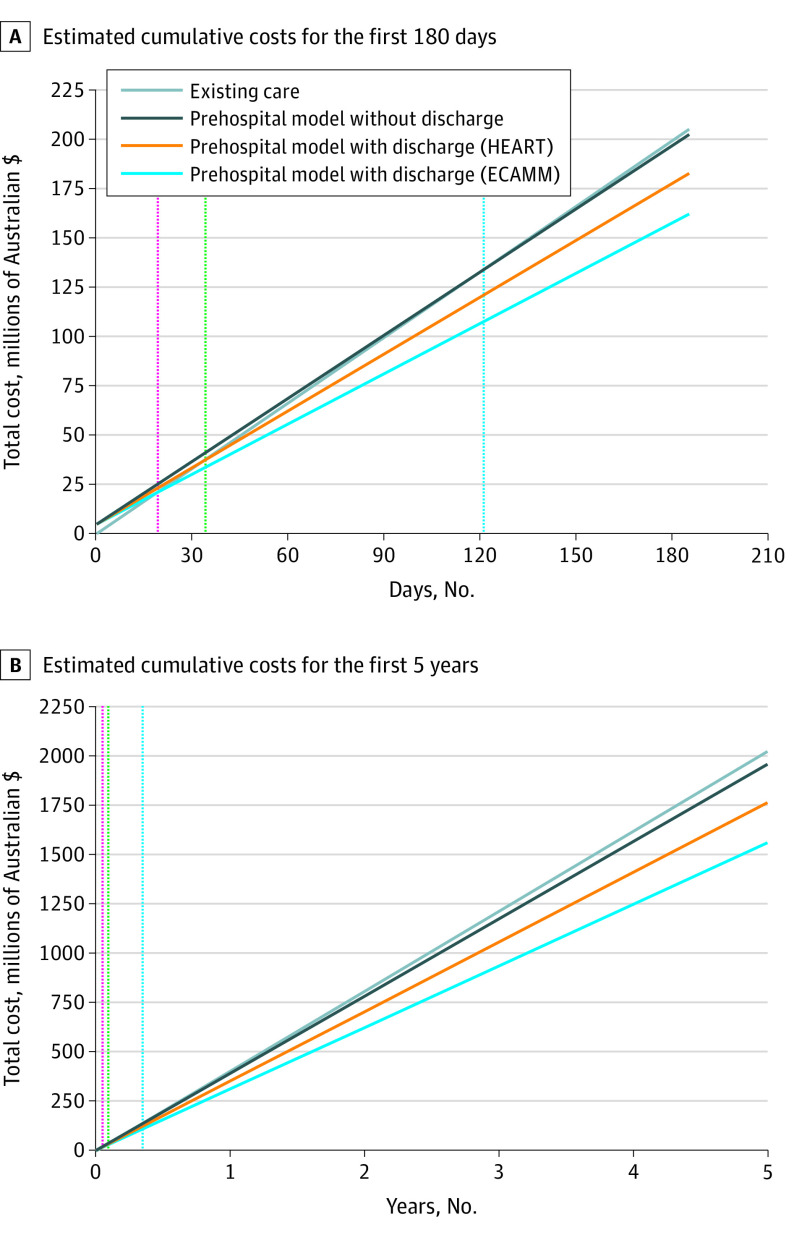
When considering point-of-care device costs to equip the ambulance fleet as an initial cost of $4.97 million rather than an annualized cost over 5 years, the break-even point was estimated at day 121 for pathway 2, day 33 for pathway 3 using the HEART score, and day 19 for pathway 3 using the ECAMM score (Figure 3). The estimated cost savings over a 5-year period in Victoria would be $64.66, $258.93, and $462.87 million for each pathway, respectively.
Figure 3. Estimated Cumulative Costs Over Time for Ambulance Attendances for Chest Pain With a Prehospital Risk Stratification and Point-of-Care Troponin Model.
Shown are estimated cumulative costs for the first 180 days, with setup costs estimated at $4.97 million assuming 60 000 chest pain presentations per year in Victoria, Australia (population at risk, 6.7 million people), and estimated cumulative costs for the first 5 years. The break-even point was 121 days for the prehospital model without prehospital discharge (dotted blue line), 33 days with prehospital discharge using the history, electrocardiogram, age, risk factors, and troponin (HEART) risk score (dotted green line), and 19 days with prehospital discharge using the Early Chest Pain Admission Mortality and Myocardial Infarction (ECAMM) score (dotted red line).
Discussion
In this economic evaluation, we performed a cost-minimization modeling analysis to assess the cost differences of using prehospital risk stratification and point-of-care troponin testing in patients attended by ambulance with acute chest pain based on assumed outcome equivalence with respect to short-term cardiac events and mortality.10 Despite initial setup costs of $4.97 million and annual costs associated with hsTn cartridges (and virtual ED staffing in the case of prehospital discharge), prehospital risk stratification and troponin measurement was estimated to result in annual cost savings of $6.45 million without using prehospital discharge and $42.84 to $71.84 million if prehospital discharge with the HEART or ECAMM risk score was used. Sensitivity analyses revealed that the risk assessment model without prehospital discharge was robust to variations in input parameters, with no thresholds identified whereby the prehospital troponin models would become more expensive than existing care, except for the model without prehospital discharge if admission rates were reduced by less than 0.2%. Cumulative cost savings over 5 years in Victoria were estimated at approximately $259 million with prehospital discharge and $65 million without prehospital discharge for low-risk patients. These data suggest that investment in prehospital risk assessment and point-of-care troponin testing is likely to be viable based on cost savings alone and should be considered by policy makers provided that safety can be confirmed in further prospective studies.
Several studies have shown that paramedic risk assessment and point-of-care troponin testing are feasible in the prehospital setting, with minimal delays in transport times to the hospital.5,6,7,8,9,10 In some studies, prehospital blood draws for immediate testing on arrival to the ED have been associated with reduced ED length of stay, results that might be extrapolated to prehospital point-of-care troponin testing.6,8 In the Netherlands, 1 trial using prehospital risk assessment and serial point-of-care troponin testing (via 2 ambulance visits) categorized 28% of the cohort as low risk, with these patients referred to other health services rather than being transported to the ED with similar outcomes.10 Several trials are under way or planned, and if the safety of nontransport for low-risk patients can be clearly established, there may be substantial benefits for health systems in reducing hospital overcrowding and ambulance offload delays and for patients in reducing assessment times, as well as reductions in costs.25,26
While the barrier costs of fleetwide point-of-care devices, paramedic training, hsTn cartridges, and virtual EDs are substantial, our study suggests that these could be outweighed by cost savings and would be recovered in the first year of operation. Importantly, we found that there may be reduced costs with prehospital risk stratification and point-of-care troponin testing, even if the current paradigm of transporting all patients to the hospital regardless of risk is maintained. The cost of virtual EDs to assess low-risk patients was borne by chest pain in our model but would have benefits for other emergency medical services and ED presentations and represents a conservative cost-savings estimate. Also important to note is that most follow-up costs after a chest pain presentation, such as local medical officer review, may occur regardless of pathway. Single-rule-out hsTn testing protocols for low-risk patients are common in many EDs, and achieving a small reduction in admission rates by patients arriving at EDs with completed point-of-care troponin tests seems reasonable, although this could be influenced by differences in local protocols, staff practices, and facilities, which were not tested in this study. In the Randomised Assessment of Treatment Using Panel Assay of Cardiac Markers (RATPAC) trial, which randomly assigned patients to point-of-care or standard protocol contemporary troponin testing in UK EDs, rates of successful discharge and median length of stay were improved, but there were no improvements in overall hospital bed use.24,27 Impacts on length of stay and cost in the RATPAC trial were beneficial at some hospitals and detrimental at others, suggesting that savings could be substantially attenuated if point-of-care testing leads to changes in local clinical processes.28
Limitations
This study has several limitations. First, data assessing paramedic-led risk stratification and point-of-care troponin testing are emerging, and while some data are available to suggest noninferiority for early cardiac events,10 the safety of nontransport to the hospital needs to be further established in larger studies. Second, the analysis assumes the use of hsTn assays rather than contemporary troponin assays, with 1 study finding that the latter were unable to safely exclude ACS in the prehospital setting.9 Similarly, this analysis assumes no change in downstream testing and does not factor in costs associated with facilities of downstream testing, which might be associated with differences such as those observed in the RAPID-TnT trial.29 Third, we assessed direct health care costs for each attendance without accounting for other fixed ambulance, ED, and hospital costs that remain present if case volume is lowered. Fourth, the ECAMM risk score for undifferentiated chest pain requires prospective validation prior to use. Fifth, while virtual EDs are used by some centers for non–chest pain cases in our region to reduce overcrowding, their proposed use as detailed in this study remains theoretical and requires further validation. Sixth, lower index attendance costs may not necessarily translate to lower annual costs if the model results in higher readmission rates following the attendance, such as that seen in a cost analysis comparing 0/1- vs 0/3-hour rapid rule-out troponin protocols.30
Conclusions
The findings of this cost-minimization study suggest that implementing ambulance-based prehospital risk stratification and point-of-care troponin testing for acute chest pain presentations may result in substantial reductions in costs. Cost savings were identified regardless of whether low-risk patients with chest pain were transported to the hospital or referred to other medical services, although savings were greater with the latter approach. These data should be considered by policy makers and emergency medical services organizations in decisions surrounding the potential utility of prehospital chest pain risk stratification and point-of-care troponin models provided that safety can be confirmed in further prospective studies.
eMethods. Supplemental Methods
eFigure 1. Cohort Derivation
eFigure 2. Univariable Sensitivity Analyses of Prehospital Risk Stratification and Point-of-Care Troponin Costs
eTable 1. Summary of Single Rule-Out High-Sensitivity Troponin (hsTn) Assay Validation Studies Presenting Data Regarding Proportion of Patients Classified as Low Risk (Acute Coronary Syndrome ruled-out) and Negative Predictive Value
eTable 2. Weighted Inlier Equivalent Separation Prices Used to Estimate Hospital Admission Costs With Adjustment for Inflation to 2020-2021 Levels
eTable 3. Costs for Each Component of Care Used to Derive Each Episode Cost
eTable 4. Cohort Characteristics
eTable 5. Episode Outcome and Disposition
eTable 6. Sensitivity of Increasing Inpatient Costs Across a Range of 10% to 30%
eTable 7. Sensitivity of Increasing Ambulance Attendance Costs for Attendances Using Prehospital Discharge Across a Range of 10% to 100%
eTable 8. Sensitivity of Increasing Costs for Patients With Suspected Acute Coronary Syndrome Who Might Be Transferred to Revascularization Centers Unnecessarily Due to False-Positive High-Sensitivity Troponin (hsTn) Values
eReferences
Data Sharing Statement
References
- 1.Cullen L, Greenslade J, Merollini K, et al. Cost and outcomes of assessing patients with chest pain in an Australian emergency department. Med J Aust. 2015;202(8):427-432. doi: 10.5694/mja14.00472 [DOI] [PubMed] [Google Scholar]
- 2.Pedersen CK, Stengaard C, Friesgaard K, et al. Chest pain in the ambulance; prevalence, causes and outcome—a retrospective cohort study. Scand J Trauma Resusc Emerg Med. 2019;27(1):84. doi: 10.1186/s13049-019-0659-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dawson LP, Smith K, Cullen L, et al. Care models for acute chest pain that improve outcomes and efficiency: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(23):2333-2348. doi: 10.1016/j.jacc.2022.03.380 [DOI] [PubMed] [Google Scholar]
- 4.Dawson LP, Andrew E, Nehme Z, et al. Incidence, diagnoses and outcomes of ambulance attendances for chest pain: a population-based cohort study. Ann Epidemiol. 2022;72:32-39. doi: 10.1016/j.annepidem.2022.04.010 [DOI] [PubMed] [Google Scholar]
- 5.Sagel D, Vlaar PJ, van Roosmalen R, et al. Prehospital risk stratification in patients with chest pain. Emerg Med J. 2021;38(11):814-819. doi: 10.1136/emermed-2020-210212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DuCharme B, Macci Bires A, Montanye E, et al. Effect of prehospital blood draws on length of stay for chest pain patients in the emergency department: a pilot study. Crit Care Nurs Q. 2019;42(2):208-214. doi: 10.1097/CNQ.0000000000000257 [DOI] [PubMed] [Google Scholar]
- 7.Ishak M, Ali D, Fokkert MJ, et al. Fast assessment and management of chest pain patients without ST-elevation in the pre-hospital gateway (FamouS Triage): ruling out a myocardial infarction at home with the modified HEART score. Eur Heart J Acute Cardiovasc Care. 2018;7(2):102-110. doi: 10.1177/2048872616687116 [DOI] [PubMed] [Google Scholar]
- 8.Stopyra JP, Snavely AC, Ashburn NP, et al. EMS blood collection from patients with acute chest pain reduces emergency department length of stay. Am J Emerg Med. 2021;47:248-252. doi: 10.1016/j.ajem.2021.04.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper JG, Ferguson J, Donaldson LA, et al. The Ambulance Cardiac Chest Pain Evaluation in Scotland Study (ACCESS): a prospective cohort study. Ann Emerg Med. 2021;77(6):575-588. doi: 10.1016/j.annemergmed.2021.01.012 [DOI] [PubMed] [Google Scholar]
- 10.Tolsma RT, Fokkert MJ, van Dongen DN, et al. Referral decisions based on a pre-hospital HEART score in suspected non-ST-elevation acute coronary syndrome: final results of the FamouS Triage study. Eur Heart J Acute Cardiovasc Care. 2022;11(2):160-169. doi: 10.1093/ehjacc/zuab109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husereau D, Drummond M, Augustovski F, et al. ; CHEERS 2022 ISPOR Good Research Practices Task Force . Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMJ. 2022;376:e067975. doi: 10.1136/bmj-2021-067975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson LP, Andrew E, Nehme Z, et al. Association of socioeconomic status with outcomes and care quality in patients presenting with undifferentiated chest pain in the setting of universal health care coverage. J Am Heart Assoc. 2022;11(7):e024923. doi: 10.1161/JAHA.121.024923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson LP, Andrew E, Stephenson M, et al. The influence of ambulance offload time on 30-day risks of death and re-presentation for patients with chest pain. Med J Aust. 2022;217(5):253-259. doi: 10.5694/mja2.51613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;144(22):e368-e454. doi: 10.1161/CIR.0000000000001030 [DOI] [PubMed] [Google Scholar]
- 15.Boeddinghaus J, Nestelberger T, Koechlin L, et al. ; APACE Investigators . Early diagnosis of myocardial infarction with point-of-care high-sensitivity cardiac troponin I. J Am Coll Cardiol. 2020;75(10):1111-1124. doi: 10.1016/j.jacc.2019.12.065 [DOI] [PubMed] [Google Scholar]
- 16.Pickering JW, Young JM, George PM, et al. Validity of a novel point-of-care troponin assay for single-test rule-out of acute myocardial infarction. JAMA Cardiol. 2018;3(11):1108-1112. doi: 10.1001/jamacardio.2018.3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wassie M, Lee MS, Sun BC, et al. Single vs serial measurements of cardiac troponin level in the evaluation of patients in the emergency department with suspected acute myocardial infarction. JAMA Netw Open. 2021;4(2):e2037930. doi: 10.1001/jamanetworkopen.2020.37930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dawson LP, Andrew E, Nehme Z, et al. Development and validation of a comprehensive early risk prediction model for patients with undifferentiated acute chest pain. Int J Cardiol Heart Vasc. 2022;40:101043. doi: 10.1016/j.ijcha.2022.101043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neumann JT, Sörensen NA, Schwemer T, et al. Diagnosis of myocardial infarction using a high-sensitivity troponin I 1-hour algorithm. JAMA Cardiol. 2016;1(4):397-404. doi: 10.1001/jamacardio.2016.0695 [DOI] [PubMed] [Google Scholar]
- 20.Chew DP, Lambrakis K, Blyth A, et al. A randomized trial of a 1-hour troponin T protocol in suspected acute coronary syndromes: the Rapid Assessment of Possible Acute Coronary Syndrome in the Emergency Department With High-Sensitivity Troponin T Study (RAPID-TnT). Circulation. 2019;140(19):1543-1556. doi: 10.1161/CIRCULATIONAHA.119.042891 [DOI] [PubMed] [Google Scholar]
- 21.Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. doi: 10.1016/j.ijcard.2013.01.255 [DOI] [PubMed] [Google Scholar]
- 22.Mahler SA, Riley RF, Hiestand BC, et al. The HEART Pathway randomized trial: identifying emergency department patients with acute chest pain for early discharge. Circ Cardiovasc Qual Outcomes. 2015;8(2):195-203. doi: 10.1161/CIRCOUTCOMES.114.001384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Six AJ, Backus BE, Kelder JC. Chest pain in the emergency room: value of the HEART score. Neth Heart J. 2008;16(6):191-196. doi: 10.1007/BF03086144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goodacre SW, Bradburn M, Cross E, Collinson P, Gray A, Hall AS; RATPAC Research Team . The Randomised Assessment of Treatment using Panel Assay of Cardiac Markers (RATPAC) trial: a randomised controlled trial of point-of-care cardiac markers in the emergency department. Heart. 2011;97(3):190-196. doi: 10.1136/hrt.2010.203166 [DOI] [PubMed] [Google Scholar]
- 25.Aarts GWA, Camaro C, van Geuns RJ, et al. Acute rule-out of non-ST-segment elevation acute coronary syndrome in the (pre)hospital setting by HEART score assessment and a single point-of-care troponin: rationale and design of the ARTICA randomised trial. BMJ Open. 2020;10(2):e034403. doi: 10.1136/bmjopen-2019-034403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Koning E, Biersteker TE, Beeres S, et al. Prehospital triage of patients with acute cardiac complaints: study protocol of HART-c, a multicentre prospective study. BMJ Open. 2021;11(2):e041553. doi: 10.1136/bmjopen-2020-041553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodacre S, Bradburn M, Fitzgerald P, et al. The RATPAC (Randomised Assessment of Treatment using Panel Assay of Cardiac markers) trial: a randomised controlled trial of point-of-care cardiac markers in the emergency department. Health Technol Assess. 2011;15(23):iii-xi, 1-102. doi: 10.3310/hta15230 [DOI] [PubMed] [Google Scholar]
- 28.Bradburn M, Goodacre SW, Fitzgerald P, et al. ; RATPAC Research Team . Interhospital variation in the RATPAC trial (Randomised Assessment of Treatment using Panel Assay of Cardiac markers). Emerg Med J. 2012;29(3):233-238. doi: 10.1136/emj.2010.108522 [DOI] [PubMed] [Google Scholar]
- 29.Lambrakis K, Papendick C, French JK, et al. Late outcomes of the RAPID-TnT randomized controlled trial: 0/1-hour high-sensitivity troponin T protocol in suspected ACS. Circulation. 2021;144(2):113-125. doi: 10.1161/CIRCULATIONAHA.121.055009 [DOI] [PubMed] [Google Scholar]
- 30.Chuang MA, Gnanamanickam ES, Karnon J, et al. Cost effectiveness of a 1-hour high-sensitivity troponin-T protocol: an analysis of the RAPID-TnT trial. Int J Cardiol Heart Vasc. 2021;38:100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Supplemental Methods
eFigure 1. Cohort Derivation
eFigure 2. Univariable Sensitivity Analyses of Prehospital Risk Stratification and Point-of-Care Troponin Costs
eTable 1. Summary of Single Rule-Out High-Sensitivity Troponin (hsTn) Assay Validation Studies Presenting Data Regarding Proportion of Patients Classified as Low Risk (Acute Coronary Syndrome ruled-out) and Negative Predictive Value
eTable 2. Weighted Inlier Equivalent Separation Prices Used to Estimate Hospital Admission Costs With Adjustment for Inflation to 2020-2021 Levels
eTable 3. Costs for Each Component of Care Used to Derive Each Episode Cost
eTable 4. Cohort Characteristics
eTable 5. Episode Outcome and Disposition
eTable 6. Sensitivity of Increasing Inpatient Costs Across a Range of 10% to 30%
eTable 7. Sensitivity of Increasing Ambulance Attendance Costs for Attendances Using Prehospital Discharge Across a Range of 10% to 100%
eTable 8. Sensitivity of Increasing Costs for Patients With Suspected Acute Coronary Syndrome Who Might Be Transferred to Revascularization Centers Unnecessarily Due to False-Positive High-Sensitivity Troponin (hsTn) Values
eReferences
Data Sharing Statement