Fig. 4 |. LNP characterization and tissue-specific Luc mRNA delivery results for 4A3-SC8-based SORT LNPs prepared by pipette mixing, vortex mixing and microfluidic mixing methods.
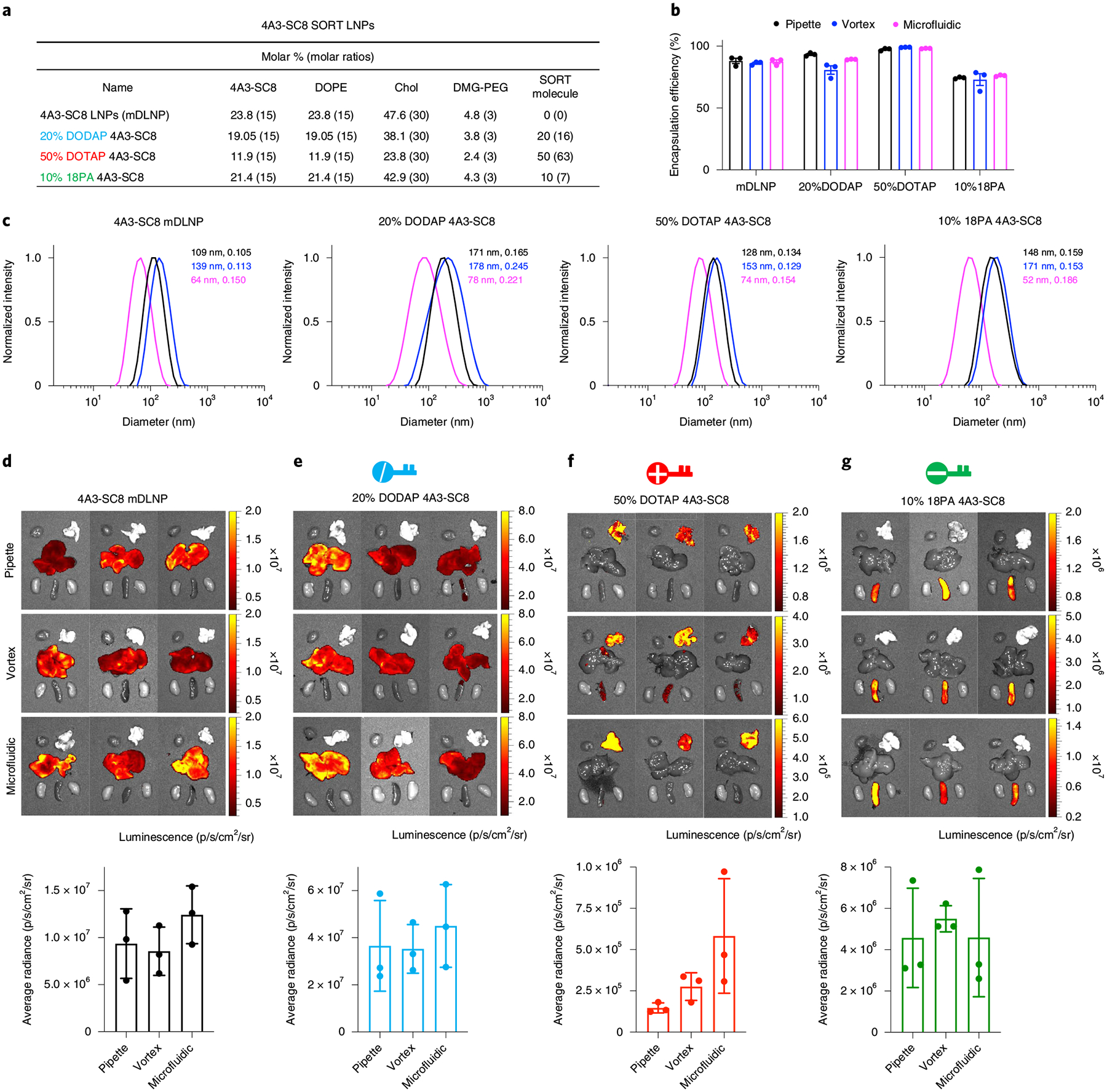
a, Formulation details of base four-component 4A3-SC8 mDLNPs and five-component 4A3-SC8 SORT LNPs. b, Encapsulation efficiency of mRNA in 4A3-SC8-based LNPs. c, Representative dynamic light scattering (DLS) analysis of 4A3-SC8-based LNPs, particle sizes and PDIs are shown. d–g, 4A3-SC8-based Luc mRNA SORT LNPs were prepared by three mixing methods and injected intravenously into mice at a dosage of 0.1 mg/kg Luc mRNA. After 6 h, ex vivo images of luminescence (from top to bottom rows: pipette, vortex and microfluidic mixing methods) in major organs (heart, lungs, liver, spleen and kidneys) and quantification data are shown. Data are presented as mean ± s.e.m. (n = 3 biologically independent animals). 4A3-SC8 SORT LNPs with 20% DODAP (e) enhanced liver delivery. Inclusion of 50% DOTAP (f) and 10% 18PA (g) shifted the luciferase protein expression to the lungs and spleen, respectively. Statistical analysis of data in Fig. 4d–g is shown in Supplementary Fig. 4. Please see the Data Availability Statement and source data for more information. All the animal experiments were approved by the IACUC of the UTSW and were consistent with local, state and federal regulations as applicable.
