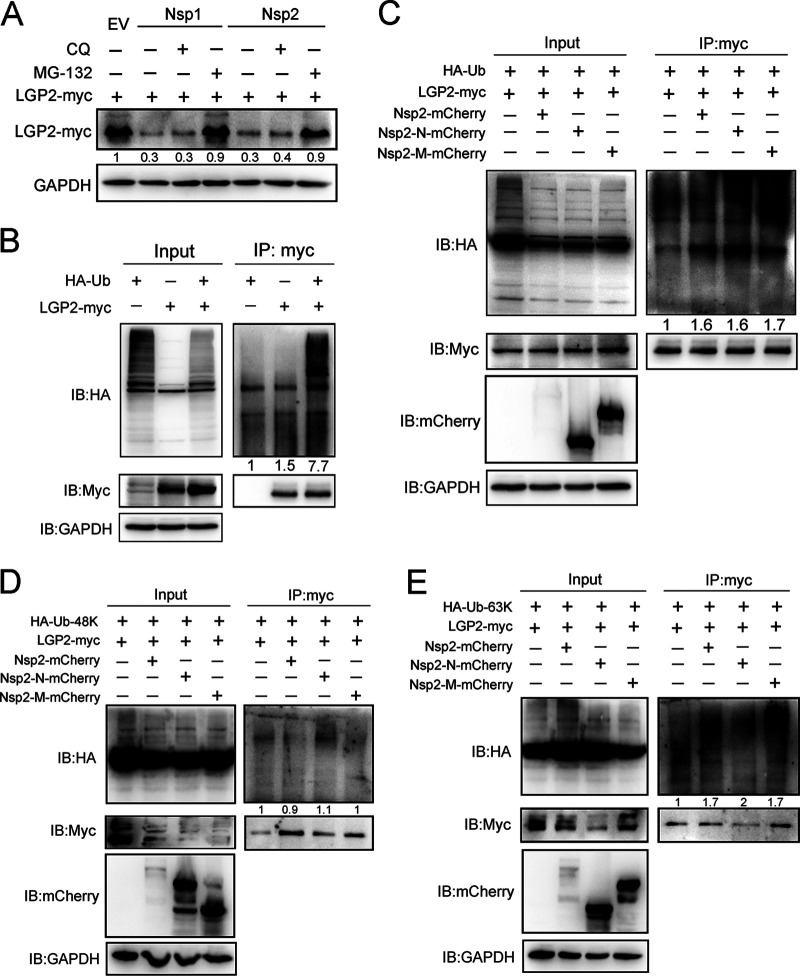
FIG 9.
PRRSV Nsp1 and Nsp2 promote LGP2 degradation via K63-linked polyubiquitination. (A) Plasmids expressing mCherry-tagged Nsp1 or Nsp2 and Myc-tagged Nsp2 were cotransfected into HEK293T cells, respectively. After 24 h of transfection, cells were mock treated or treated with chloroquine (CQ; 50 μM) and MG132 (10 μM) for 6 h. Cells were lysed for Western blotting using Myc antibody. GAPDH was included as an internal control. (B) HEK293T cells were transfected with HA-tagged ubiquitin or Myc-tagged LGP2 alone or cotransfected with these two plasmids. After 24 h of transfection, cells were treated with MG132 (10 μM) for another 6 h. Cell lysates were immunoprecipitated with a Myc antibody, and immunoblots of HA and Myc are shown. (C) HEK293T cells were cotransfected with plasmids expressing Myc-tagged LGP2 and mCherry-tagged Nsp2 or its truncations, together with HA-tagged ubiquitin. After 24 h, the cells were treated with MG132 (10 μM). Co-IP was used to detect the ubiquitination level of LGP2 using an antibody against Myc. Immunoblots are shown using antibodies against HA, Myc, mCherry, and GAPDH. (D) HEK293T cells were transfected with mCherry-tagged Nsp2 or other Nsp2 fragments with Myc-tagged LGP2 and HA-tagged K48-linked ubiquitin for 24 h and were treated with MG132 (10 μM) for another 6 h. Cell lysates were immunoprecipitated with a Myc antibody, and immunoblots of HA, Myc, and mCherry are shown using the indicated antibodies. (E) HEK293T cells were transfected with mCherry-tagged Nsp2 or other Nsp2 fragments with Myc-tagged LGP2 and HA-tagged K63-linked ubiquitin for 24 h, followed by the treatment of MG132 (10 μM). Cells were lysed for co-IP using an antibody against Myc, and immunoprecipitates were analyzed by Western blotting with HA, Myc, and mCherry antibodies. GAPDH was shown as an internal control. For the Western blot, the relative band density was normalized to the loading control GAPDH and then compared to the corresponding control.