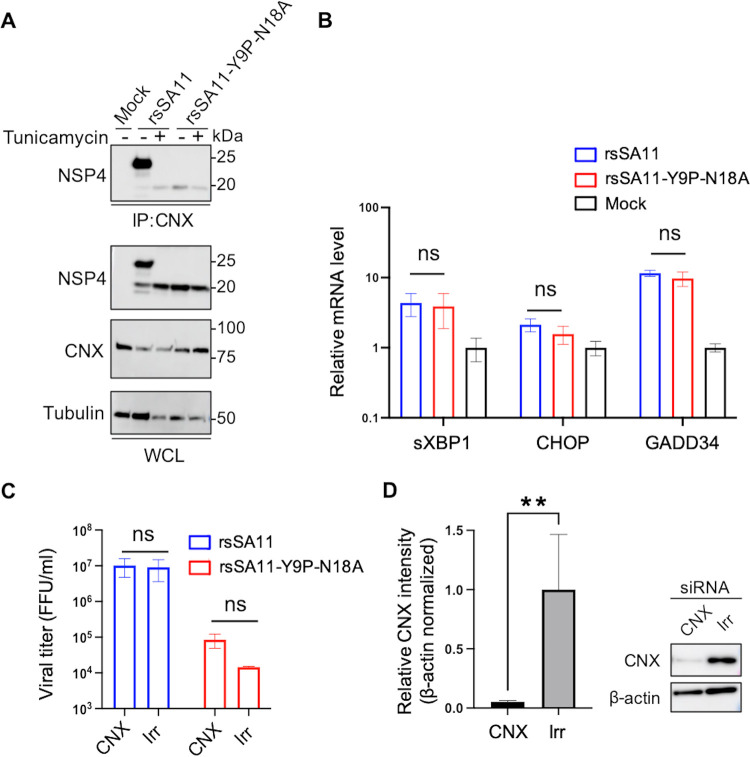
FIG 7.
N-glycosylation is required for interaction with calnexin; however, it does not affect viral replication in HT29 cells. (A) NSP4-calnexin interaction. HT29 cells were infected with rsSA11 or rsSA11-Y9P-N18A at an MOI of 5 in the presence or absence of tunicamycin. Cell lysates were collected at 18 hpi and subjected to coimmunoprecipitation with an anti-calnexin antibody. The molecular weight (kDa) is shown on the right. IP, immunoprecipitation; WCL, whole-cell lysate; CNX, calnexin. (B) UPR-related genes. HT29 cells were infected with rsSA11 or rsSA11-Y9P-N18A at an MOI of 5. At 8 hpi, cell lysates were collected and subjected to qRT-PCR. The relative mRNA levels (compared to mock samples) are shown (mock sample was set as 1). The data are expressed as means ± the SD of triplicate samples. The statistical significance was determined using a t test, with P < 0.05 considered significant (*, P < 0.05). (C and D) siRNA-mediated knockdown of calnexin. HT29 cells were transfected with siRNA targeting calnexin (CNX) or GFP (irrelevant control, Irr). At 48 h posttransfection, the cells were infected with viruses at an MOI of 0.01. At 24 hpi, cells were freeze-thawed and subjected to viral titration. The mock-infected cells were subjected to Western blotting. Relative intensity of calnexin bands was measured using imageJ software. Expression of calnexin was normalized to that of β-actin. The data are expressed as means ± the SD of triplicate samples. Statistical significance was determined using a t test, with P < 0.05 considered significant (**, P < 0.01).