Summary
Neuromodulation is a key therapeutic tool for clinicians managing patients with drug-resistant epilepsy. Multiple devices are available with long-term follow-up and real-world experience. The aim of this review is to give a practical summary of available neuromodulation techniques to guide selection of modalities, focusing on patient selection for devices, common approaches and techniques for initiation of programming, and outpatient management issues.
Vagus nerve stimulation (VNS), deep brain stimulation of the anterior nucleus of the thalamus (DBS-ANT), and responsive neurostimulation (RNS) are all supported by randomized controlled trials that show safety and a significant impact on seizure reduction, as well as a suggestion of reduction in the risk of sudden unexplained death from epilepsy (SUDEP). Significant seizure reductions are observed after 3 months for DBS, RNS, and VNS in randomized controlled trials, and efficacy appears to improve with time out to 7-10 years of follow-up for all modalities, albeit in uncontrolled follow-up or retrospective studies. A significant number of patients experience seizure-free intervals of 6 months or more with all three modalities. Number and location of epileptogenic foci are important factors affecting efficacy, and together with co-morbidities such as severe mood or sleep disorders, may influence choice of modality. Programming has evolved – DBS is typically initiated at lower current/voltage than used in the pivotal trial while charge density is lower with RNS, but generalizable optimal parameters are yet to be defined. Non-invasive brain stimulation is an emerging stimulation modality, although currently not widely used.
Clinical practice has evolved from those established in pivotal trials. Guidance is available for clinicians wishing to expand their approach, and choice of neuromodulation technique may be tailored to individual patients based on their epilepsy characteristics, risk tolerance, and preferences.
Keywords: Neuromodulation, VNS (vagus nerve stimulation), DBS (deep brain stimulation), RNS (responsive stimulation), brain stimulation
1. Introduction
Epilepsy that is refractory to anti-seizure medications remains a significant worldwide health burden.1 Resective or ablative surgery provides the greatest chance for seizure freedom, however many patients with epilepsy do not have a single, identifiable seizure focus that is suitable for a resective or ablative procedure.2 Neuromodulation (which we will use synonymously with “neurostimulation”) offers an alternative treatment approach for these non-surgical candidates.3
In addition to recently published long-term follow-up data from pivotal trials, there is also a growing body of hands-on experience and expanding clinical practice that may be helpful to the practicing clinician. The aim of this review is to provide a practical guide for neurologists and epileptologists seeking to familiarise themselves with these devices and techniques.
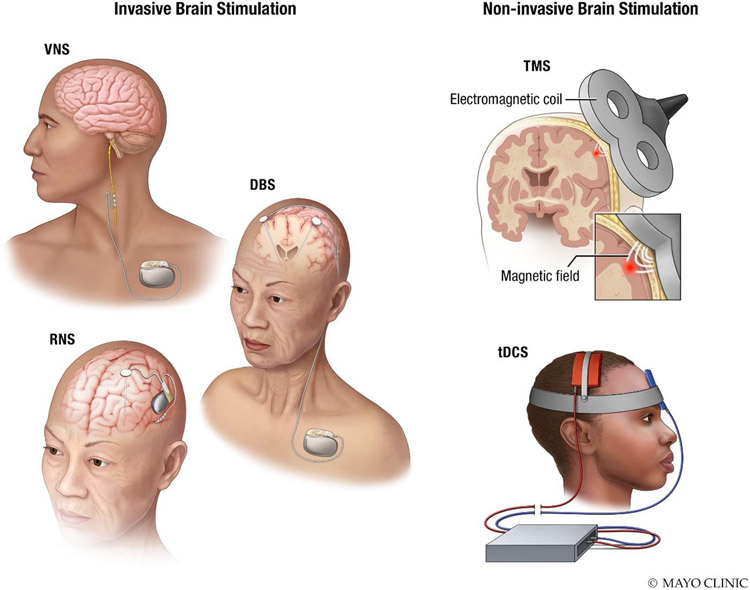
The review is structured in four parts. The first three parts address invasive neurostimulation considerations (pre-implant, implant, and post-implant), focusing on the three methods that have been approved for use in epilepsy in most major jurisdictions – vagus nerve stimulation (VNS), deep brain stimulation of the anterior nucleus of the thalamus (DBS-ANT), and responsive neurostimulation) RNS (Fig. 1A). The fourth part considers non-invasive brain stimulation (NIBS) for epilepsy (Fig. 1B).
Figure 1. Brain stimulation techniques for epilepsy.
Left: Primary approved invasive epilepsy neuromodulation techniques: VNS, DBS, and RNS. Right: Primary non-invasive brain stimulation techniques: transcranial magnetic stimulation (TMS), and transcranial direct current electrical stimulation (tDCS).
VNS devices intermittently stimulate the left vagus nerve, and are thought to exert effects via diffuse projections from the brainstem, resulting in blood flow and neurotransmitter changes, particularly in the thalamus.4,5 DBS-ANT also typically involves intermittent stimulation, but uses depth electrodes to target bilateral ANT, which forms part of the circuit of Papez and has projections to frontal and temporal cortical regions.6,7 RNS is distinct in that it allows for chronic intracranial recording from seizure network foci using cortical strips or depth electrodes and delivers responsive stimulation based on real-time EEG data.8
2. Invasive neurostimulation: pre-implant considerations
Efficacy, epilepsy type, and history
Efficacy of neuromodulation has been reported extensively and will be briefly summarized here. VNS, DBS-ANT, and RNS were found to be effective during a 3-month blinded evaluation period (mean or median seizure reduction of 25% - 28% for VNS;9-11 median of 40% for DBS-ANT, and 38% for RNS6,8). Efficacy improved with time over several years (up to 52-76% for VNS12,13, and around 75% for both DBS-ANT and RNS14,15), noting that these longer term results are from uncontrolled/unblinded observational studies (Table 1). At these later time points, the differences in degree of improvement in seizure frequencies for the three modalities may not be statistically significant. Controlled comparisons have yet to be performed. With all three modalities a significant number of patients achieve at least 6 months of seizure freedom. For DBS-ANT and RNS this was studied prospectively, and seizure-free intervals of at least 6 months were found in 18% for DBS-ANT15, and in 28% for RNS14. For VNS, only retrospective data is available, making direct comparisons less meaningful, but in one large registry study & systematic review a (terminal) rate of seizure-freedom of 8% was found.16 Other retrospective studies of VNS have reported a wide range of seizure-freedom rates using varying definitions (7-20%12,13,16-19), which is higher than that seen with increasing medication trials (approximately 4% become seizure-free on third and subsequent medication regimes, if the first two are unsuccessful 20). All modalities had a small number of patients who worsened after implantation, defined as a >50% increase in seizure frequency (VNS 3-4%11,21, DBS-ANT 3%22, RNS 5-8%14,23).
Table 1.
Device regulatory approvals US & EU
| Manufacturer(s) | Approved ages |
FDA approval |
Year approved |
CE Mark | Year approved |
|
|---|---|---|---|---|---|---|
| VNS | Cyberonics / LivaNova | ≥4yrs | Focal* epilepsy | 2017 (latest), 1997 (original) | Focal and generalized epilepsy | 2018 (latest), 1994 (original) |
| DBS-ANT | Medtronic | ≥18 | Focal* epilepsy, ≥6 seizures / month | 2018 | Focal epilepsy | 2010 |
| RNS | Neuropace | ≥18 | Focal* epilepsy with one or two foci*, ≥3 seizures / month | 2013 | N/A | N/A |
No invasive neurostimulation is FDA approved in the US for the treatment of generalized epilepsy. VNS is considered a ‘possibly effective’ treatment for focal and generalized epilepsy, and LGS, in children (2013 AAN guideline).26
VNS may be effective for both focal and generalized epilepsy, and has a European CE Mark for both indications, but is FDA-approved in the US only for focal epilepsy5,24-26. DBS-ANT has approval in both jurisdictions for focal epilepsy, while RNS is approved for focal epilepsy in the US only. While not yet approved in any major jurisdiction, DBS has a growing body of evidence supporting possible efficacy in generalized and/or multifocal epilepsies, mostly using the centromedian-parafascicular complex (CM-Pf) of the thalamus (CMT) as a target.27-35 Results from four controlled trials have been published on DBS-CMT have been encouraging but mixed;30,33-35 showing either no difference between stimulation on and stimulation off conditions 34,35 or differences for generalized but not frontal epilepsies30; or, in the most recent study, a double-bind randomized controlled trial of DBS-CMT in LGS, significant reductions in electrographic seizures, but not diary reported seizures.33 RNS has also been employed to target CMT in generalized epilepsy, however published literature is limited to case reports and small case series.36
VNS is the only FDA-approved modality in the USA for use in children (ages ≥4 years), whereas DBS-ANT and RNS are FDA approved for adults (≥18 years). VNS is considered a ‘possibly effective’ treatment for focal and generalized epilepsy, and Lennox-Gastaut Syndrome (LGS), in children (2013 AAN guideline).26 At present, stimulation for pediatric patients includes many approaches that are not FDA-approved.37 VNS has the additional benefit of being approved for the treatment of depression.
Electroclinical characteristics and efficacy
The SANTE (stimulation of the anterior nucleus of the thalamus for epilepsy) pivotal trial for DBS-ANT was powered for overall seizure reduction and only the subgroup with temporal lobe seizures had sufficient sample size to show a significant effect of stimulation.6 In long-term follow-up beyond two years, frontal lobe seizures also significantly improved, but the efficacy of ANT stimulation for parietal, occipital and multifocal seizures remains unclear.15,22 For RNS, subgroup analyses were performed both prospectively and retrospectively, however reductions in seizure frequency did not vary in a statistically significant manner between different lobes of onset, or between those with one or two onset foci.8,38,39 Lesional neocortical cases tended to fare better than non-lesional neocortical cases.39
Prior epilepsy surgery does not appear to change efficacy for any modality. For VNS, those with prior surgery were included in at least one randomized study, but subgroup analysis was not performed11. A later retrospective study did not find an association of outcomes with prior epilepsy surgery.19 In both the DBS-ANT and RNS trials, seizure reductions did not depend on prior VNS placement, nor on previous epilepsy surgery.8,40,41
Rates of probable or definite SUDEP appear to be lower than might otherwise by expected in patients treated with neurostimulation. In the RNS trial the rate was 2.8 per 1000 patient years (95% CI 1.2-6.7),14 while for DBS-ANT it was 2.0 (95% CI 0.24 – 7.12).15 For VNS, a comparable cohort study found an unadjusted rate of 4.1 per 1000 patient years, or 1.7 per 1000 patient years when adjusted for age and duration of therapy42. A more recent retrospective review found an unadjusted rate of 2.3 per 1000 patient years, or 1.7 per 1000 patient years when adjusted for age and duration of VNS therapy43. These rates for all three modalities are lower than typical rates for drug-resistant epilepsy patients being considered for surgery (4.6-9.3 per 1000 patient years),44,45 and comparable to rates achieved by successful epilepsy surgery in general (1.9 per 1000 patient years)44. However, no prospective trial has been completed comparing SUDEP rates of any neurostimulation modality to conventional medical therapy.
Diagnostic evaluation
The diagnostic evaluation for device-based neuromodulation is similar to that for epilepsy surgery candidates in general. Intracranial monitoring is not specifically required but may be helpful if a clear understanding of the seizure network is not possible without invasive monitoring. In particular, RNS requires the seizure onset zone to be clearly identified. In the original RNS study 59% of patients had invasive monitoring prior to implantation8, while in subsequent studies that rate increased to 82%;46 reflecting either an increased preference for sEEG prior to implantation, or an increased use of RNS in patients who were studied with sEEG but not considered suitable for focal resection. sEEG potentially can serve three purposes: (1) Determining whether resective surgery is likely to be a preferable first-line therapy; (2) Localizing where to place permanent electrodes in relation to seizure foci; (3) Suggesting to what extent particular thalamic nuclei are part of a seizure propagation network.
Perioperative risk
Operative and perioperative risks vary among the modalities. The lowest risk option is typically considered to be VNS, the significant risks of which are largely limited to superficial infection, found in around 3-6% of patients; vocal cord paralysis and bradycardia/asystole are rare.5 For DBS-ANT the risk profile is similar to DBS implantations for movement disorders, where intracranial hemorrhage and infection are the main concerns, though serious complications are rare. At implantation, there were asymptomatic hemorrhages detected following 4.5% implants on routine neuroimaging.6 After 10 years of follow-up in the SANTE study, implant site infection occurred in 12.7% of patients (1-year rate 7.3%), and misplaced leads requiring revision in 8.2% of patients.15 For RNS, intracranial hemorrhage rates occurred in 4.7% (3.1% peri-operative, half of which required evacuation; 1.6% associated with seizure-related head trauma).8 No hemorrhage resulted in permanent neurologic deficits. In long-term follow-up, non-seizure-related hemorrhages occurred in 2.7% of patients (all asymptomatic), and infection occurring in 12.1% of patients (4.1% per procedure).14 Device infection may necessitate device and / or lead replacement or removal, but can occasionally be managed conservatively.
3. Invasive neurostimulation: implant considerations
Battery life and primary cell vs rechargeable
VNS and RNS utilize primary cell (i.e., non-rechargeable) devices, while DBS systems use either primary cell or rechargeable batteries. Non-rechargeable devices are more convenient, but larger because of the greater battery capacity required, and necessitate more frequent replacement. Rechargeable devices require recharging, typically once or a few times per week. Forgetting to do so can cause loss of efficacy, so that greater patient compliance is required with these devices. As expected, battery depletion depends strongly on settings, with higher voltages/amperages and longer duty cycles (on periods) reducing primary cell battery life or time between charges for rechargeable devices. For primary cell devices, most new devices will provide a warning when interrogated if battery depletion is expected to occur in the near future, although the precise end of battery life can be difficult to predict due to non-linear battery depletion.
Older generation VNS devices had a median battery life of 4-6 years typically (range 2 – 8 years)12,47-49. Current models are estimated to have similar lifespans (4-8 years), but depend additionally upon the use of the tachycardia detection and closed loop stimulation feature (‘Autostim’), which shortens battery life by an estimated 1-2 years (Table 3).50
Table 3.
Battery life estimates and additional features for major devices and models
| Manufacturer | Model | Manufacturer estimates |
Real world findings |
Additional features | |
|---|---|---|---|---|---|
| VNS | Cyberonics | 100, 101, 102, 102R | 4-12 years | 2-8 years12,47-49 | Guided programming (M102, M102R only)† |
| Cyberonics | AspireHC (M105) | 7-8 years* | - | Higher capacity battery, guided programming† | |
| Cyberonics | AspireSR (M106) | 7-8 years,* 5 years*,# | - | Higher capacity battery, guided programming, † Autostim | |
| LivaNova | SenTiva (M1000) | 4-5 years, * 3-4 years*,# | - | Guided programming, Autostim, Day/night programming mode, smaller form factor | |
| DBS-ANT | Medtronic | Activa PC | - | 3 years (DBS-ANT),15 3.9 (3.3-4.5) years (PD).114 | - |
| Medtronic | Percept PC | >5 years (PD)51 | TBD | Limited sensing | |
| Medtronic | Activa RC | 9-15 years | - | Rechargeable | |
| RNS | Neuropace | 300M | 3.9 (2.6 - 4.3) years52 | 3.5 years14 | - |
| Neuropace | 320M | 8.4 (5.1 – 9.4) years53 | TBD | MRI conditional |
Using standard target settings of current output 2.0 mA, frequency 20 Hz, pulse width 250 us, and duty cycle of 33-35%.50
With Autostim enabled; 168 Autostims/day, duration 60 s.50
Requires M2000/M3000 programming wand/clinician programmer.50 TBD – to be determined. PD – using estimates from DBS for Parkinson’s disease in movement disorders.
DBS systems (Medtronic models Kinetra initially, and later Activa PC) using the SANTE study parameters have a reported median battery life of 3.0 years15. The time to replacement is anticipated to be longer for newer models,51 but this is yet to be established. A rechargeable DBS system also is available (Medtronic Activa RC) which typically needs to be charged every 1-2 weeks, for 30-60 minutes per session, and has an estimated life of 9-15 years. While the Medtronic DBS system is the only FDA-approved system for epilepsy, other manufacturers have comparable systems which may be used off-label (e.g. Boston Scientific Vercise - primary cell / Vercise Genus - rechargeable, Abbott/St. Jude Medical Infinity – primary cell).
For RNS, the older model device (Neuropace RNS 300M) was found to have a median time to replacement of 3.5 years in long-term follow-up,14 close to the manufacturers estimate of 3.9 years of battery life (range 2.6 - 4.3 years),52 whereas the newer model (Neuropace RNS 320) is estimated to have 8.4 years of battery life (range 5.1 – 9.4 years).53
Sensing: open and closed loop systems
The ability of devices to detect seizures (or seizure-related activity) is highly desirable. This activity can be used to estimate seizure burden,54 as subjective reports are inaccurate;55 to perform responsive “closed loop” stimulation;56 and to assist in seizure localization for possible further surgical treatment.57,58 Current devices have a range of abilities to detect seizures or surrogates.
Current VNS models with the ‘Autostim’ feature can detect tachycardia, and deliver a custom (typically increased) current, pulse width, and duration of stimulation, with the aim to terminate seizures early in their course. Hence seizures associated with early tachycardia are an attractive target for VNS. Heart rate detection is accurate and the responsive stimulation is well-tolerated.59,60 Retrospective data suggest patients may experience an additional seizure reduction after replacement with tachycardia-sensing VNS devices – 71% of patients experienced an additional ≥50% seizure reduction (compared to the initial response to VNS).61
The Medtronic Percept implantable pulse generator (IPG) allows for recording of the local field potential (LFP, local EEG) at ANT or any implanted site. At the present time, raw EEG only can be viewed live by use of the programmer in a medical setting. Percept does not store raw EEG but only the LFP power spectrum for a 5 Hz band centered on a frequency of choice over a 10-minute duration. This (chronic) sensing feature must be enabled on two contacts surrounding the referential stimulating cathodal contact(s). The spectral peaks could be correlated with events marked in the patient log. In principle, asymmetry of power spectrum might indicate a left versus right temporal seizure;62 however, 5-10 second epoch durations, which are not currently available in Percept, are required for accurate seizure detection. Hence it is not presently a responsive or closed loop system.
Unlike VNS and DBS, RNS is designed primarily as a closed-loop stimulation system. Patients receive a laptop and interrogation wand with their device, and are encouraged to connect and upload their data daily, or every few days. ECoG data recorded from the intracranial leads can then be viewed directly using a web-based interface, and detectors can be programmed and customized to record interictal and ictal activity. Stimulation is delivered responsively with detections, according to stimulation settings. The relative value of responsive stimulation during a seizure versus a chronic neuromodulatory effect has not been established. RNS devices may stimulate several thousands of times per day, with the vast majority of stimulations unassociated with a behaviorally-evident or EEG-evident seizure. This might be sufficient to provide a long-term neuromodulatory effect,63 and would be consistent with the observed increasing benefit over time.
A unique benefit of RNS is the ability to discriminate between two hypothesized seizure onset zones in terms of targets for resective surgery. For example, in bitemporal epilepsy, long- term monitoring may reveal that one temporal lobe generates the vast majority of seizures, whereas on short term inpatient monitoring sessions it may have appeared that seizure were equally distributed.57,58 Resective or ablative procedures on the predominant temporal lobe can then be re-considered.
Targeting and pre-operative neuroimaging
Accurate targeting is an important consideration for DBS-ANT and RNS implantations. Direct visualization of the ANT (more specifically, AV – the anteroventral nucleus64, sometimes called the principal nucleus) is possible using standard T1 weight MR imaging (e.g. MPRAGE - magnetisation-prepared rapid gradient echo), short tau inversion recovery (STIR), or white matter nulled T1 imaging (e.g. FGATIR – Fast Gray matter Acquisition T1 Inversion Recovery). Some practitioners have argued that the ideal ANT target is in AV just superior to the termination of the mammillothalamic tract.65 For DBS-ANT, a transventricular approach has been the standard approach and was used in the original SANTE study. An anterior extraventricular approach appears to be less accurate,66 while a posterior extraventricular approach appears to be safe and accurate.67 For RNS targeting bilateral hippocampal structures with depth electrodes for mesial temporal epilepsy, it was found that even leads near but not within the hippocampus were effective.38
4. Invasive neurostimulation: post-implant considerations
Initiation and subsequent follow-up
Stimulation is often initiated at the first outpatient visit, which is typically 2-4 weeks following implantation, in part to allow for the implantation effect to subside. Subsequent visits may be every 1-6 months (typically every 3-4 months), and then eventually annually or less frequently when programming is stable. Common to all outpatient visits is system interrogation, which involves checking battery levels and lead impedances. For RNS, a company representative is typically present or available by phone for assistance with programming and to provide suggested detection settings. For VNS and DBS this is not usually required at programming visits, once the programmer has gained familiarity with the device.
Initiation and titration of VNS
For VNS, the original randomized controlled studies started stimulation 2 weeks post-implant, and the physicians’ manual stipulates that device current must be 0 mA for the first 14 days after implantation. Three groups of settings must be programmed for the newest models: (1) basic duty cycle stimulation (‘Normal mode’), (2) ‘Autostim’: custom stimulation triggered by heart rate detection, and (3) ‘Magnet mode’: similar to Autostim but triggered by manual swipes of a magnet over the IPG by the patient (e.g., for a presumed seizure). The SenTiva (model M1000) also has a ‘day/night’ programming mode, where two different groups of settings can be used over a 24-hour cycle. The initial phase of programming involves slow up-titration in increments of 0.125 or 0.25 mA to a current of 1.5-2 mA, using a pulse width of 250 us and frequency of 20 Hz (Table 4). This is followed by a second phase of reducing duty cycle OFF time, aiming for a duty cycle of 16-58% (depending on tolerance and use of Autostim).
Table 4.
Typical stimulation settings for VNS, DBS, and RNS
| Current, voltage, or charge density |
Pulse width |
Frequency | Duty cycle %, ON/OFF (min) |
|||||
|---|---|---|---|---|---|---|---|---|
| Initial | Increment | Target | Initial | Target | ||||
| VNS | Normal mode | 0.125 - 0.25 mA | 0.125 - 0.25 mA | 1.5-2 mA | 250 μs | 20-30 Hz | 10%, 0.5 ON / 5 OFF | 16-58% |
| Autostim | 0.25 - 0.375 mA | 0.125 - 0.25 mA | 1.5-2 mA | 250 μs | 20-30 Hz | ON 1 | ON 1 | |
| Magnet mode | 0.5 mA | 0.25 mA | 2 mA | 500 μs | 20-30 Hz | ON 1 | ON 1 | |
| DBS (ANT) | SANTE protocol | 2-3 mA/V per cathode | 1-2 mA/V | 5-6 mA/V | 90 μs | 145 Hz | 17%, 1 ON / 5 OFF | |
| RNS | Initial | 0.5 μC/cm2 | 0.5 μC/cm2 | 2-6 μC/cm2 | 160 μs | 200 Hz | ||
One approach to setting the Autostim threshold is to start with a 20-40% threshold and aim for 50-100 Autostims per day. Up-titration may either be done manually, with changes made every 1-2 weeks for 7-8 visits, or programmed to occur automatically (‘guided mode’), available in newer models. Side effects are often encountered during up-titration. Common strategies when side effects are encountered include reducing pulse width or frequency. If this fails, then reductions in current are likely required, usually by 0.125 or 0.25 mA. In general, patients need hours to days to become accustomed to voice changes and coughing, which are common when amplitudes are increased. Most or all of the throat symptoms decline with slow up-titration of current and continued usage, however hoarseness of the voice frequently remains perceivable during stimulation periods. If throat-related side effects prevent amplitude titration to at least 1 mA, lowering stimulation frequency to 10-15 Hz and/or reducing pulse width to 125 μs can facilitate tolerance of increased amplitudes. Once 1 mA has been reached, sometimes patients can then tolerate later increases in frequency or pulse width. In general, increases in amplitude are prioritized over stimulation frequency or pulse width.
Initiation and titration of DBS
The initial visits for DBS-ANT in the pivotal trial were at 4 weeks, however in practice this is done as early as 2 weeks. There are no specific contraindications to starting stimulation immediately after implantation, and in European centers this is a common practice, often conjoined with inpatient monitoring for a few days. All new DBS systems are current-clamped. Current typically starts at 2-3 mA per cathode, with an aim to increase in increments of 1-2 mA every 1-3 months up to around 5 mA per cathode or until efficacy is achieved (Table 4). Often a single referential (‘monopolar’) electrode configuration is used, ideally selected based on post-implantation imaging. If the electrode impedance is 1,000 Ohms, then 5 mA is equivalent to 5 V, which was the amplitude used in the SANTE trial with voltage-clamped devices. If two or more cathodes are enabled in referential configuration on the Percept system, it is important to be aware that each contact will deliver the set number of mA, instead of dividing the mA by number of cathodes, as was done with the Activa system.
No specific parameters have been demonstrated to be superior to others in prospective or retrospective reviews of data, however a flowchart has been developed based on expert consensus, which may aid in titration and troubleshooting.68 For example, if response is suboptimal despite increasing current, a second cathode may be added (typically while reducing overall current by 30-50%). Cathode location can also be changed, preferably guided by neuroimaging. Other options include reducing OFF time in duty cycle or trying a different frequency. Strategies for when side effects are encountered (including an increase in habitual seizures or new seizures) include reducing current amplitude by 30-50% (or by 1-2 mA/V), reducing frequency, or changing to bipolar configurations (e.g., 1-3 cathodes per lead, 1 anode per lead). DBS patient programmers allow for patients to make changes at home, e.g., under the supervision of physician via a telemedicine visit or via pre-determined instructions.
In general, frequencies above 100 Hz have been used for DBS-ANT, and there has been concern that lower frequencies (e.g. <45 Hz) could worsen seizures.69 However, stimulation frequencies between 3 and 40 Hz have been used for DBS-ANT and DBS-CM.27 In addition, DBS hardware has been used to deliver chronic subthreshold low frequency stimulation to cortical targets with promising results.70,71 Pulse widths up to 500 μs have been used,68 and we typically consider 6-7 mA per lead to be a reasonable upper limit, although occasionally up to ~10 mA or 10 V have been used.
Initiation and titration of RNS
RNS stimulation typically is initiated at around 4 weeks, after multiple seizures have been recorded. Passive recording of several seizures aids in setting the patient-specific detection algorithms for responsive stimulation, which include line length, half wave, and area algorithms. Detection after implantation commonly uses the line-length method, capitalizing on the added length of the EEG tracing with large amplitude delta activity, spikes, or low amplitude high frequency activity during a seizure. When a characteristic frequency for the seizures is determined, the detection method usually is switched to half wave, to detect power increases around that frequency, which usually results in faster detections. Ongoing seizure detections allow for further refinements in detection and stimulation parameters, aiming to detect seizures within 0.5 s of onset.
Electrodes can be configured in multiple ways, but a ‘stacked bipolar’ or ‘checkerboard’ (+−+−) pattern of alternating cathodes/anodes is often useful. Current is typically initiated to achieve a charge density of 0.5 μC/cm2 and incremented by the same amount every 3-4 months (Table 4). Typical charge density targets are approximately 2 μC/cm2 for mesial temporal structures,38 and perhaps somewhat higher (around 6 μC/cm2) for neocortical targets.39 In a recent retrospective review, there was a general trend noted towards a lower charge density (approximately 2 μC/cm2 rather than 4-5 μC/cm2 ), and lower current (3.5 mA versus 4.5 mA),72 compared to the pivotal RNS trial. There was also an increased duration of stimulation of 4.5 min/day, which is in line with some clinicians’ preference to target a certain number of stimulations per day (e.g., 1000 therapies per day), but still far less than the duty cycling modalities of VNS and DBS (approximately 2 – 12 hours).
Regarding stimulation parameters, bursts of 200 Hz (or 200 pulses per seconds) for 100 msec are typically used. A pulse width of 160 microseconds is typically used. Lower stimulation frequencies may sometimes be helpful, and although most published data for low frequency cortical stimulation is limited to non-responsive approaches,70,71,73 emerging evidence suggests that some who do not respond to high frequency stimulation (>45 Hz), may respond to low frequency stimulation (7-40 Hz).74 Electrode configuration can also be changed to referential (‘monopolar’) using 1-4 cathodes on each lead. In addition, RNS allows for regional (lead-to-lead) stimulation,75 which produces a broader field than standard configurations. Finally, increasing detector sensitivity can reduce the latency to seizure detection and increase number of therapies per day, both of which may improve outcomes.
Stimulation-related side effects
Most patients with VNS experience some voice alteration, typically hoarseness, during stimulation, particularly at initiation (up to 66% in the first 3 months5,10), and after increasing current amplitude at programming sessions; and many habituate somewhat over hours to days.5,76 Cough, dyspnea, pain, paresthesia, & headache also are common at 3 months (16-25%), but decline at 12 months (13-16%), and become uncommon by 5 years (1-5%).5 An important side effect not anticipated in clinical trials, but found in clinical practice, is sleep-disordered breathing and worsening of obstructive sleep apnea, which may persist despite CPAP therapy.77-79 Differential day/night programming have help to ameliorate this.
Depression and memory impairment have been reported with DBS-ANT, mostly in those who had a prior history of these symptoms. In the SANTE study at 5 years, 37% of patients reported depression, and 11.8% reported suicidal ideation,22 while neurocognitive outcomes were mixed. Memory impairment was reported by 27%, with 1/3 confirmed to have a change from baseline neuropsychometric testing, although other neuropsychological outcomes improved.22 While these percentages remained stable out to 7 years following implantation,15 a separate analysis of the data concluded that these mood and cognitive changes did not result in significant long-term objective neurobehavioral changes.80 For some patients, notable depressive symptoms, anxiety, and even psychosis can develop over days or more insidiously with DBS-ANT stimulation.81 Reducing stimulation amplitude, changing the electrode configuration, or otherwise altering stimulation parameters has been noted to alleviate or completely resolve these symptoms. Stimulation-induced paresthesias are relatively common and can be alleviated by lowering the current or switching from a referential to bipolar electrode configuration. New stimulation-induced seizure types are rare, but have been reported.6 Approximately 3% of patients had a 50% increase in seizure frequency at 5 years.22 Sleep was not studied prospectively in the pivotal trial, but limited evidence suggests that sleep cycle disruption may be another side effect of DBS-ANT.82
Symptoms of depression and memory complaints were commonly reported at baseline in the RNS pivotal study as well as during the trial period.8 At baseline, 49% had a history of depression and 53-56% had objective memory dysfunction. By two years, depressive symptoms were reported by 13.6%, with suicidality in 6.8%. Neuropsychometric testing did not show a decline memory or cognition but rather improvements in some areas (language and memory mostly, with some dependence on the seizure onset zone/stimulated region).8,83 At follow-up to 9 years, 23.4% of participants reported at least mild depression, 1.6% reported a serious adverse event related to depression, and suicidality was reported in 9.8%.14 Mild memory impairment was reported by 12.5% and 1 out of 256 patients had a serious adverse event related to memory. Similar to DBS-ANT, the main risk factors for reporting mood and memory symptoms were their presence prior to implantation. Approximately 5% of patients experienced at least a 50% increase in seizure frequency. Stimulation-induced sensory effects were occasionally encountered, including photopsia in 14.4% of mesial temporal RNS patients,38 and again these can usually be resolved with stimulation parameter changes. Sleep was not studied prospectively, but at least one report did not find an association between RNS and sleep disturbance.84
Managing devices for MRI, surgery, and other procedures
MRIs can produce excessive heating of device components, leading to tissue or device damage. Devices are often switched off or placed in a safe mode for surgical procedures and MRI. The newest VNS, DBS, and RNS devices are all MRI-conditional, having met FDA-specified requirements for the scans. Not all legacy devices may be safe for scanning.85 Head CT scans are allowable with all neurostimulators, although cranial implants produce artifacts on both CT and MR imaging. During surgery the main concern is electrocautery and the potential for tissue and device damage.
Certain therapeutic and diagnostic procedures are contraindicated with some devices, such as transcranial magnetic stimulation (TMS), transcranial direct current stimulation (tDCS), diathermy, therapeutic ultrasound, and electroconvulsive therapy (ECT). Reference to manufacturer manuals is recommended Cardiac defibrillators can damage chest neurostimulators, but might be required in an emergency.86 All implanted devices come with a warning against high current or voltage activities, such as arc-welding, and require extra attention at airport security.
Device removal & replacement
Removal and replacement of the IPG due to battery depletion is the most common indication for repeat device surgery and is typically straightforward. One complexity can arise when a voltage-clamped system (e.g., Activa PC) is replaced with a current clamped system (e.g., Percept PC). In this case, therapy current (i.e. the total current the device uses to deliver therapy) and electrode impedances should be measured prior to replacement and used to guide selection of new current settings.
Stimulating electrodes (and extensions) can be difficult to remove safely and may be left in place after IPG removal. For example, VNS electrode removal can lead to nerve or vessel injury. These ‘abandoned leads’ require special attention for MRI scans and should be noted for local surgery and electrocautery. Heating of isolated wires during an MRI potentially can cause tissue injury. MRI can be performed with residual disconnected VNS leads provided that the length of the VNS lead is less than two cm from the proximal to distal contact along the vagus nerve.
5. Non-invasive stimulation for epilepsy
Non-invasive brain stimulation may offer advantages as a low risk and cost-effective means for treating drug-resistant epilepsy. For both transcutaneous vagus nerve stimulation (tVNS) and transcutaneous trigeminal nerve stimulation (TNS), CE-certified devices are available in Europe intended for treatment of epilepsy. In contrast, non-invasive stimulation using repetitive transcranial magnetic stimulation (rTMS) systems and devices for transcranial direct current stimulation (tDCS) are used off-label in both outpatient and inpatient settings (e.g. rTMS for refractory focal status epilepticus).
tVNS uses bipolar electrical stimulation of cutaneous sensory fibers of the vagus nerve in the medial fossa of the auricular concha. Stimulation is performed at frequencies of 10-25 Hz at intensities below the individual perception threshold (0.6-1 mA). Efficacy data are available for two stimulators, a German “Nemos” stimulator, CE-certified in Europe; and a Chinese TENS-200 stimulator. Reductions in seizure frequency have been reported in small studies applying bilateral tVNS at 20 Hz ,87 while a study using left-sided stimulation with the Nemos stimulator at 25 Hz did not find significance superiority vs control stimulation at 2 Hz.88 Hence while tVNS is well-tolerated - infrequent adverse effects include local skin irritation, dizziness, otalgia, nasopharyngitis, and headaches - evidence for its efficacy remains incomplete.89
TNS is applied bilaterally and supraorbitally over the ophthalmic nerve (V1), usually overnight. A randomized controlled phase II trial lasting 18 weeks compared an active group stimulated at 120 Hz and a control group stimulated at 2 Hz (30 s on, 30 s off, pulse width of 250 s, intensity below perception threshold, for 12 hours per day).90 Neither seizure frequency, responder rate, nor time to fourth seizure showed significant changes between treatment groups, though there was a responder rate of 40.5% after 18 weeks within the 120 Hz stimulation group which dropped to 36.8% after 12 months. Several subsequent open-label treatment reports found very modest (11-35%) improvements in seizure frequency and rapidly declining adherence. TNS tolerability was very good, adverse effects consisted mainly of local skin irritation and headache. Overall, clinical evidence for the efficacy of TNS is limited, and further study is needed before determining that it is efficacious.
rTMS has been applied at frequencies of 0.1 to 1 Hz to induce long-term depression of cortical excitability.91 Most studies measure inhibitory effects of rTMS by means of paired pulses producing thumb twitches, with a smaller second twitch reflecting greater inhibition. This assay only applies to motor cortex. Measurement of rTMS-evoked EEG potentials can assess inhibition at any neocortical location.92 After an initial uncontrolled report of an antiseizure effects of rTMS at 0.33 Hz following daily stimulation sessions with a round coil over the vertex,93 several sham-stimulation-controlled studies failed to reproduce this effect (with stimulation applied over the vertex or over the area of the epileptic focus), until more recently rTMS at 0.5 Hz and 90% resting motor potential over the epileptic focus was shown to be effective in a controlled trial.94 rTMS has also been used acutely in status epilepticus in case reports and small case series, and appears to be effective (though often only transiently).95,96 Prolonged changes in EEG biomarkers and long-term seizure freedom have also been observed in response to short-term rTMS.97 European evidence-based guidelines concluded that evidence for efficacy of rTMS in epilepsy was lacking,98 and a recent Cochrane review found that although rTMS was safe and reduced epileptiform discharges, also noted a paucity of evidence for efficacy.99
tDCS is performed with a cathode placed over the epileptogenic area and the anode contralaterally or over frontal regions. Cathodal stimulation locally hyperpolarizes cortical pyramidal neurons and exerts additional longer lasting inhibitory effects. Stimulation is usually performed for 20-30 minutes at intensities of 1 or 2 mA.100tDCS for treatment of epilepsy has been studied in numerous sham-controlled and uncontrolled prospective studies.101-104 Controlled studies resulted in a reduction in seizure frequency by 44-89% during the follow-up period, which was typically one to three months after stimulation. Interictal epileptiform EEG activity was also reduced in some studies,104 however this has not been consistently reported.101 Newer approaches to tailoring tDCS therapy include sEEG-based targeting of the seizure onset zone (rather than via scalp EEG or imaging alone),103 and the use of multielectrode montages further shape the electric field of stimulation.102 Thus while tDCS is safe and may be effective, study methodology and protocols have varied greatly, and evidence-based recommendations remain guarded.105 An implantable device capable of transcranial DC-like stimulation of the epileptic focus is presently under investigation.106
6. Conclusions and future directions
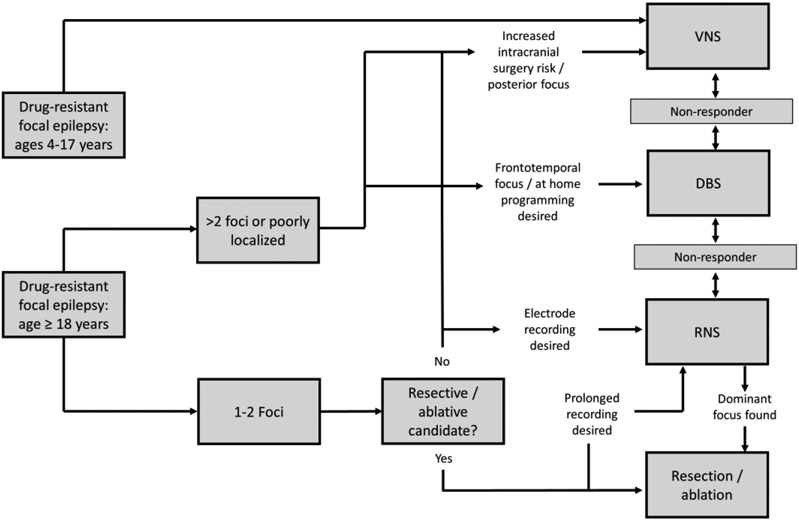
A wide range of factors may influence the decision to pursue neuromodulation for individual patients and influence the choice of neuromodulation system (Fig. 2 & Table 5). Programming has evolved, most notably to begin with lower current/voltage than in the pivotal trials. Clinical approaches are increasingly incorporating a variety of targets related to both focal and generalized epilepsies,30,33,107,108 the ability to sense/record intracranial data chronically and stimulate responsively,109 as well as new programing waveforms and stimulation approaches.70,110,111 More study is required to determine optimal stimulation parameters, ideally personalized for individual patients, and biomarkers or other predictors of likely stimulation responders. Evidence indicates that existing stimulation methods should be considered as a form of palliative therapy, and are not a substitution for more definitive resective or ablative therapy when these treatment can be safely utilized.
Figure 2. Suggested algorithm for selection of invasive neuromodulation technique.
The primary invasive neuromodulation modalities are included for children (age 4 – 17 years) and adults (≥18 years) who have failed two trials of anti-seizure medications.
Table 5.
Factors influencing choice of neuromodulation
| VNS | RNS | DBS | ||
|---|---|---|---|---|
| Epilepsy type | ||||
| Oligofocal (1-2 foci) | + | + | + | |
| Multifocal (>2 foci) or diffuse | + | − | ? | |
| Generalized | + | ? | ? | |
| Lobe of onset | ||||
| Anterior: frontal/temporal | + | + | + | |
| Posterior: parietal/occipital | + | + | ? | |
| Not well-localized | + | − | + | |
| Comorbidity | ||||
| Severe depression / suicidality | + | + | − | |
| Significant sleep disorder | − | ? | ? | |
| Significant cardiac rhythm abnormality | − | + | +/−* | |
| Clinician preference | ||||
| Chronic intracranial recording desirable | − | + | − | |
| Prefer lower programming burden | + | − | + | |
| Ability for at-home changes | − | − | + | |
| Patient preference | ||||
| Low risk tolerance | + | − | − | |
| Prefer chest IPG | + | − | + | |
| Prefer cranial IPG | − | + | − |
Key: + favorable, − less favorable, ? unknown; for each modality.
Depending on likelihood of DC cardioversion being required.
Table 2.
General efficacy: seizure reduction and responder rates
| 3mo | 1yr | 2yr | 3yr | 5yr | Long term (7-15yr) |
||
|---|---|---|---|---|---|---|---|
| Therapy | Outcome | ||||||
| VNS | |||||||
| Responder rate | 23-31%9-11 | 31-49%16,21,112,113 | 43%112 | 43-63%16,112 | - | 68-86%12,19 | |
| Median (or mean)* reduction in seizure freq. | 25-28%9-11 | 26-52%12,13,16,21,112,113 | 44-58%12,112 | 44-63%12,16,112 | 30-60%12,13 | 52-76%12,13 | |
| 6-month seizure-free intervals | - | 5%16 | - | 8%16 | - | - | |
| 12-month seizure-free intervals | - | - | - | - | - | 7-20%12,13,16-19 | |
| ≥50% increase in seizures | 4%11 | 3%21 | - | - | - | - | |
| DBS | |||||||
| Responder rate | - | 43%6 | 54%6 | 67%6 | 68%22 | 74%15 | |
| Median reduction in seizure freq. | 40%6 | 41%6 | 56%6 | - | 69%22 | 75%15 | |
| 6-month seizure-free intervals | - | - | - | 13%6 | 16%22 | 18%15 | |
| 12-month seizure-free intervals | - | - | - | 7%6 | - | - | |
| ≥50% increase in seizures | - | - | - | - | 3%22 | - | |
| RNS | |||||||
| Responder rate | 29%8 | 43%8 | 46%8 | 58-61%23 | 50-61%23 | 73%14 | |
| Median reduction in seizure freq. | 38%8 | 44%23 | 53%23 | 60-66%23 | 48-66%23 | 75%14 | |
| 6-month seizure-free intervals | - | - | - | - | 23%23 | 28%14 | |
| 12-month seizure-free intervals | - | - | - | - | 13%23 | 18%14 | |
| ≥50% increase in seizures | - | - | - | - | 8%23 | 5%14 |
3-month data is for the prospective, blinded, and controlled studies, while later timepoints include unblinded/uncontrolled and retrospective studies. All seizure-freedom rates for VNS are from uncontrolled retrospective studies, hence there is a wide range of values reported.
Seizure reductions are quoted as medians where available, and means where medians not available.
Key points.
Multiple devices are approved for invasive brain stimulation for epilepsy with long-term follow-up data and real-world experience
Practical recommendations are provided for choosing stimulation modalities, programming devices, and managing patients with devices
Initial programming has evolved to start at lower voltage/current amplitudes
Non-invasive brain stimulation for epilepsy is a promising modality but currently has limited evidence for efficacy
Disclosures & funding
Declarations of interest
Hugh Simpson has no conflicts of interest.
Andreas Schulze-Bonhage has obtained research support from UNEEG and Precisis.
Gregory Cascino has no conflicts of interest.
Robert Fisher has relevant disclosures of Medtronic consulting and disclosures not relevant to this article include stock or options in Avails Medical, Cerebral Therapeutics, Irody, Smart-Monitor, Zeto.
Barbara Jobst has support as the Louis and Ruth Frank Professor of Neurosciences and as Associate Editor of Neurology. She has received research support from Neuropace, Inc., Harvard Pilgrim Inc., NIH and CDC.
Michael Sperling has received compensation for speaking at CME programs from Medscape, Projects for Knowledge, International Medical Press, and Eisai. He has consulted for Medtronic, Neurelis, and Johnson & Johnson. He has received research support from Eisai; Medtronic; Neurelis; SK Life Science; Takeda; Xenon; Cerevel; UCB Pharma; Janssen; and Engage Pharmaceuticals. He has received royalties from Oxford University Press and Cambridge University Press
Brian Lundstrom declares intellectual property licensed to Cadence Neuroscience Inc (contractual rights waived), site investigator (Medtronic EPAS, NeuroPace RESPONSE, Neuroelectrics tDCS for Epilepsy), and industry consultant (Epiminder, Medtronic, Philips Neuro; money to Mayo Clinic). He was funded by the NIH NINDS (K23NS112339).
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
Ethical publication statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Fiest KM et al. Prevalence and incidence of epilepsy. Neurology 88, 296–303 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thijs RD, Surges R, O’Brien TJ & Sander JW Epilepsy in adults. Lancet 393, 689–701 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Sprengers M, Vonck K, Carrette E, Marson AG & Boon P Deep brain and cortical stimulation for epilepsy. Cochrane database Syst. Rev 7, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binnie CD Vagus nerve stimulation for epilepsy: A review. Seizure 9, 161–169 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Ben-Menachem E Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurology vol. 1 477–482 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Fisher R et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51, 899–908 (2010). [DOI] [PubMed] [Google Scholar]
- 7.Li MCH & Cook MJ Deep brain stimulation for drug-resistant epilepsy. Epilepsia vol. 59 273–290 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Morrell MJ Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology 77, 1295–1304 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Ben-Menachem E et al. Vagus Nerve Stimulation for Treatment of Partial Seizures: 1. A Controlled Study of Effect on Seizures. Epilepsia 35, 616–626 (1994). [DOI] [PubMed] [Google Scholar]
- 10.Handforth A et al. Vagus nerve stimulation therapy for partial-onset seizures: A randomized active-control trial. Neurology 51, 48–55 (1998). [DOI] [PubMed] [Google Scholar]
- 11.George R et al. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology 45, 224–230 (1995). [DOI] [PubMed] [Google Scholar]
- 12.Elliott RE et al. Efficacy of vagus nerve stimulation over time: Review of 65 consecutive patients with treatment-resistant epilepsy treated with VNS > 10 years. Epilepsy Behav. 20, 478–483 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Uthman BM et al. Effectiveness of vagus nerve stimulation in epilepsy patients. Neurology 63, 1124–1126 (2004). [DOI] [PubMed] [Google Scholar]
- 14.Nair DR et al. Nine-year prospective efficacy and safety of brain-responsive neurostimulation for focal epilepsy. Neurology (2020) doi: 10.1212/WNL.0000000000010154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salanova V et al. The SANTÉ study at 10 years of follow-up: Effectiveness, safety, and sudden unexpected death in epilepsy. Epilepsia 62, 1306–1317 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Englot DJ, Rolston JD, Wright CW, Hassnain KH & Chang EF Rates and Predictors of Seizure Freedom With Vagus Nerve Stimulation for Intractable Epilepsy. Neurosurgery 79, 345–353 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghaemi K et al. Vagus nerve stimulation: Outcome and predictors of seizure freedom in long-term follow-up. Seizure 19, 264–268 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Janszky J et al. Vagus nerve stimulation: predictors of seizure freedom. J. Neurol. Neurosurg. Psychiatry 76, 384–389 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wasade VS et al. Long-term seizure and psychosocial outcomes of vagus nerve stimulation for intractable epilepsy. Epilepsy Behav. 53, 31–36 (2015). [DOI] [PubMed] [Google Scholar]
- 20.Kwan P & Brodie MJ Early identification of refractory epilepsy. N. Engl. J. Med (2000) doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 21.DeGiorgio CM et al. Prospective Long-Term Study of Vagus Nerve Stimulation for the Treatment of Refractory Seizures. Epilepsia 41, 1195–1200 (2000). [DOI] [PubMed] [Google Scholar]
- 22.Salanova V et al. Long-term efficacy and safety of thalamic stimulation for drug-resistant partial epilepsy. Neurology 84, 1017–1025 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergey GK et al. Long-term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology (2015) doi: 10.1212/WNL.0000000000001280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satya-Murti S, Shepard KM & Helmers SL Vagus nerve stimulation in the treatment of epilepsy. Neurol. Clin. Pract 3, 431–435 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labar D, Murphy J & Tecoma E Vagus nerve stimulation for medication-resistant generalized epilepsy. Neurology 52, 1510–1512 (1999). [DOI] [PubMed] [Google Scholar]
- 26.Morris GL et al. Evidence-based guideline update: Vagus nerve stimulation for the treatment of epilepsy. Neurology 81, 1453–1459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alcala-Zermeno JL et al. Centromedian thalamic nucleus with or without anterior thalamic nucleus deep brain stimulation for epilepsy in children and adults: A retrospective case series. Seizure 84, 101–107 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Velasco F et al. Electrical Stimulation of the Centromedian Thalamic Nucleus in Control of Seizures: Long-Term Studies. Epilepsia 36, 63–71 (1995). [DOI] [PubMed] [Google Scholar]
- 29.Cukiert A, Cukiert CM, Burattini JA & Mariani PP Seizure outcome during bilateral, continuous, thalamic centromedian nuclei deep brain stimulation in patients with generalized epilepsy: a prospective, open-label study. Seizure 81, 304–309 (2020). [DOI] [PubMed] [Google Scholar]
- 30.Valentín A et al. Deep brain stimulation of the centromedian thalamic nucleus for the treatment of generalized and frontal epilepsies. Epilepsia 54, 1823–1833 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Velasco F et al. Centromedian Nucleus and Epilepsy. J. Clin. Neurophysiol 38, 485–493 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Vetkas A et al. Deep brain stimulation targets in epilepsy: Systematic review and meta-analysis of anterior and centromedian thalamic nuclei and hippocampus. Epilepsia 00, 1–12 (2022). [DOI] [PubMed] [Google Scholar]
- 33.Dalic LJ et al. DBS of thalamic centromedian nucleus for Lennox–Gastaut syndrome ( ESTEL trial). Ann. Neurol 4 (2021) doi: 10.1002/ana.26280. [DOI] [PubMed] [Google Scholar]
- 34.Velasco F et al. Predictors in the treatment of difficult-to-control seizures by electrical stimulation of the centromedian thalamic nucleus. Neurosurgery 47, 295–305 (2000). [DOI] [PubMed] [Google Scholar]
- 35.Fisher RS et al. Placebo-Controlled Pilot Study of Centromedian Thalamic Stimulation in Treatment of Intractable Seizures. Epilepsia 33, 841–851 (1992). [DOI] [PubMed] [Google Scholar]
- 36.Burdette DE et al. Brain-responsive corticothalamic stimulation in the centromedian nucleus for the treatment of regional neocortical epilepsy. Epilepsy Behav. 112, 107354 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Starnes K, Miller K, Wong-Kisiel L & Lundstrom BN A Review of Neurostimulation for Epilepsy in Pediatrics. Brain Sci. 2019, Vol. 9, Page 283 9, 283 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geller EB et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia 58, 994–1004 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Jobst BC et al. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia 58, 1005–1014 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Fisher RS et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 58, 522–530 (2017). [DOI] [PubMed] [Google Scholar]
- 41.Parisi V et al. Anterior Nucleus of the Thalamus Deep Brain Stimulation with Concomitant Vagus Nerve Stimulation for Drug-Resistant Epilepsy. Neurosurgery 89, 686–694 (2021). [DOI] [PubMed] [Google Scholar]
- 42.Annegers JF, Coan SP, Hauser WA & Leestma J Epilepsy, Vagal Nerve Stimulation by the NCP System, All-Cause Mortality, and Sudden, Unexpected, Unexplained Death. Epilepsia 41, 549–553 (2000). [DOI] [PubMed] [Google Scholar]
- 43.Ryvlin P et al. Long-term surveillance of SUDEP in drug-resistant epilepsy patients treated with VNS therapy. Epilepsia 59, 562–572 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Casadei CH et al. All-cause mortality and SUDEP in a surgical epilepsy population. Epilepsy Behav. 108, 107093 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomson T, Nashef L & Ryvlin P Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol. 7, 1021–1031 (2008). [DOI] [PubMed] [Google Scholar]
- 46.Razavi B et al. Real-world experience with direct brain-responsive neurostimulation for focal onset seizures. Epilepsia 61, 1749–1757 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vonck K et al. Generator replacement in epilepsy patients treated with vagus nerve stimulation. Seizure 14, 89–99 (2005). [DOI] [PubMed] [Google Scholar]
- 48.Couch JD, Gilman AM & Doyle WK Long-term Expectations of Vagus Nerve StimulationA Look at Battery Replacement and Revision Surgery. Neurosurgery 78, 42–46 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Révész D, Rydenhag B & Ben-Menachem E Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. J. Neurosurg. Pediatr 18, 97–104 (2016). [DOI] [PubMed] [Google Scholar]
- 50.LivaNova USA. VNS therapy system. Epilepsy physician’s manual. US Version. May 2020. vol. 5 https://dynamic.cyberonics.com/manuals/emanual_download.asp?lang=English-US&docid=%7BBAA7EE19-92D5-4E78-8480-5BB49CD87744%7D (2021). [Google Scholar]
- 51.Medtronic. Percept PC Neurostimulator. https://www.medtronic.com/us-en/healthcare-professionals/products/neurological/deep-brain-stimulation-systems/percept-pc.html (2021). [Google Scholar]
- 52.Neuropace. RNS System User Manual - 300M. https://www.neuropace.com/wp-content/uploads/2021/02/rns-system-manual-300m.pdf (2019). [Google Scholar]
- 53.Neuropace. RNS System Physician Manual - RNS-320. https://www.neuropace.com/wp-content/uploads/2021/02/neuropace-rns-system-manual-320.pdf (2020). [Google Scholar]
- 54.Quigg M et al. Electrocorticographic events from long-term ambulatory brain recordings can potentially supplement seizure diaries. Epilepsy Res. 161, 106302 (2020). [DOI] [PubMed] [Google Scholar]
- 55.Karoly P, Goldenholz DM & Cook M Are the days of counting seizures numbered? Curr. Opin. Neurol 31, 162–168 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Vassileva A, van Blooijs D, Leijten F & Huiskamp G Neocortical electrical stimulation for epilepsy: Closed-loop versus open-loop. Epilepsy Research vol. 141 95–101 (2018). [DOI] [PubMed] [Google Scholar]
- 57.King-Stephens D et al. Lateralization of mesial temporal lobe epilepsy with chronic ambulatory electrocorticography. Epilepsia 56, 959–967 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirsch LJ et al. Mesial temporal resection following long-term ambulatory intracranial EEG monitoring with a direct brain-responsive neurostimulation system. Epilepsia 61, 408–420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boon P et al. A prospective, multicenter study of cardiac-based seizure detection to activate vagus nerve stimulation. Seizure 32, 52–61 (2015). [DOI] [PubMed] [Google Scholar]
- 60.Fisher RS et al. Automatic Vagus Nerve Stimulation Triggered by Ictal Tachycardia: Clinical Outcomes and Device Performance—The U.S. E-37 Trial. Neuromodulation Technol. Neural Interface 19, 188–195 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamilton P et al. Clinical outcomes of VNS therapy with AspireSR® (including cardiac-based seizure detection) at a large complex epilepsy and surgery centre. Seizure 58, 120–126 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Gregg NM et al. Anterior nucleus of the thalamus seizure detection in ambulatory humans. Epilepsia 62, e158–e164 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruzzone MJ et al. Insights into the therapeutic effect of responsive neurostimulation assessed with scalp EEG recording: A case report. J. Clin. Neurophysiol 35, 438–441 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Jones EG The Thalamus. (Cambridge University Press, 2007). [Google Scholar]
- 65.Schaper FLWVJ et al. Deep Brain Stimulation in Epilepsy: A Role for Modulation of the Mammillothalamic Tract in Seizure Control? Neurosurgery 87, 602–610 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lehtimäki K et al. The Surgical Approach to the Anterior Nucleus of Thalamus in Patients With Refractory Epilepsy: Experience from the International Multicenter Registry (MORE). Neurosurgery 84, 141–150 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Wang YC et al. Targeting analysis of a novel parietal approach for deep brain stimulation of the anterior nucleus of the thalamus for epilepsy. Epilepsy Res. 153, 1–6 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Fasano A et al. Experience and consensus on stimulation of the anterior nucleus of thalamus for epilepsy. Epilepsia 62, 2883–2898 (2021). [DOI] [PubMed] [Google Scholar]
- 69.Yu T et al. High-frequency stimulation of anterior nucleus of thalamus desynchronizes epileptic network in humans. Brain 141, 2631–2643 (2018). [DOI] [PubMed] [Google Scholar]
- 70.Lundstrom BN, Gompel J. Van, Khadjevand F, Worrell G & Stead M Chronic subthreshold cortical stimulation and stimulation-related EEG biomarkers for focal epilepsy. Brain Commun. 1, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lundstrom BN et al. Chronic Subthreshold Cortical Stimulation to Treat Focal Epilepsy. JAMA Neurol. 73, 1370–1372 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Razavi B et al. Real-world experience with direct brain-responsive neurostimulation for focal onset seizures. Epilepsia 61, 1749–1757 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lim SN et al. Low and High Frequency Hippocampal Stimulation for Drug-Resistant Mesial Temporal Lobe Epilepsy. Neuromodulation Technol. Neural Interface 19, 365–372 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alcala-Zermeno JL et al. Low Frequency Stimulation in Patients with Responsive Neurostimulation. AES 2021 Annual Meeting Abstract Database. AESnet.org. (2021). [Google Scholar]
- 75.Ma BB et al. Responsive neurostimulation for regional neocortical epilepsy. Epilepsia 61, 96–106 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Englot DJ, Chang EF & Auguste KI Vagus nerve stimulation for epilepsy: a meta-analysis of efficacy and predictors of response: A review. J. Neurosurg 115, 1248–1255 (2011). [DOI] [PubMed] [Google Scholar]
- 77.Oh DM et al. Treatment of vagus nerve stimulator-induced sleep-disordered breathing: A case series. Epilepsy Behav. Reports 12, 100325 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parhizgar F, Nugent K & Raj R Obstructive Sleep Apnea and Respiratory Complications Associated with Vagus Nerve Stimulators. J. Clin. Sleep Med 7, 401–407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oliveira Santos M et al. Complex sleep-disordered breathing after vagus nerve stimulation: broadening the spectrum of adverse events of special interest. Epileptic Disord. 22, 790–796 (2020). [DOI] [PubMed] [Google Scholar]
- 80.Tröster AI, Meador KJ, Irwin CP & Fisher RS Memory and mood outcomes after anterior thalamic stimulation for refractory partial epilepsy. Seizure 45, 133–141 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Järvenpää S et al. Reversible psychiatric adverse effects related to deep brain stimulation of the anterior thalamus in patients with refractory epilepsy. Epilepsy Behav. 88, 373–379 (2018). [DOI] [PubMed] [Google Scholar]
- 82.Voges BR et al. Deep brain stimulation of anterior nucleus thalami disrupts sleep in epilepsy patients. Epilepsia 56, e99–e103 (2015). [DOI] [PubMed] [Google Scholar]
- 83.Loring DW, Kapur R, Meador KJ & Morrell MJ Differential neuropsychological outcomes following targeted responsive neurostimulation for partial-onset epilepsy. Epilepsia 56, 1836–1844 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Ruoff L et al. Sleep disruption is not observed with brain-responsive neurostimulation for epilepsy. Epilepsia Open 5, 155–165 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watson RE & Edmonson HA MR Safety: Active Implanted Electronic Devices. Magn. Reson. Imaging Clin 28, 549–558 (2020). [DOI] [PubMed] [Google Scholar]
- 86.Medtronic. Indications, safety, and warnings - Deep Brain Stimulation Therapy. https://www.medtronic.com/us-en/healthcare-professionals/therapies-procedures/neurological/deep-brain-stimulation/indications-safety-warnings.html. [Google Scholar]
- 87.Aihua L et al. A controlled trial of transcutaneous vagus nerve stimulation for the treatment of pharmacoresistant epilepsy. Epilepsy Behav. 39, 105–110 (2014). [DOI] [PubMed] [Google Scholar]
- 88.Bauer S et al. Transcutaneous Vagus Nerve Stimulation (tVNS) for Treatment of Drug-Resistant Epilepsy: A Randomized, Double-Blind Clinical Trial (cMPsE02). Brain Stimul. 9, 356–363 (2016). [DOI] [PubMed] [Google Scholar]
- 89.Lampros M, Vlachos N, Zigouris A, Voulgaris S & Alexiou GA Transcutaneous Vagus Nerve Stimulation (t-VNS) and epilepsy: A systematic review of the literature. Seizure 91, 40–48 (2021). [DOI] [PubMed] [Google Scholar]
- 90.DeGiorgio CM et al. Randomized controlled trial of trigeminal nerve stimulation for drug-resistant epilepsy. Neurology 80, 786–791 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen R et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48, 1398–1403 (1997). [DOI] [PubMed] [Google Scholar]
- 92.EEG Evoked R et al. EEG Evoked Potentials to Repetitive Transcranial Magnetic Stimulation in Normal Volunteers: Inhibitory TMS EEG Evoked Potentials. Sensors 2022, Vol. 22, Page 1762 22, 1762 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tergau F, Naumann U, Paulus W & Steinhoff BJ Low-frequency repetitive transcranial magnetic stimulation improves intractable epilepsy. Lancet 353, 2209 (1999). [DOI] [PubMed] [Google Scholar]
- 94.Sun W et al. Low-frequency repetitive transcranial magnetic stimulation for the treatment of refractory partial epilepsy: A controlled clinical study. Epilepsia 53, 1782–1789 (2012). [DOI] [PubMed] [Google Scholar]
- 95.Liu A, Pang T, Herman S, Pascual-Leone A & Rotenberg A Transcranial magnetic stimulation for refractory focal status epilepticus in the intensive care unit. Seizure 22, 893–896 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stavropoulos I, Pak HL & Valentin A Neuromodulation in Super-refractory Status Epilepticus. J. Clin. Neurophysiol 38, 494–502 (2021). [DOI] [PubMed] [Google Scholar]
- 97.Starnes K et al. Case Report: Prolonged Effects of Short-term Transcranial Magnetic Stimulation on EEG Biomarkers, Spectral Power, and Seizure Frequency. Front. Neurosci 783 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lefaucheur JP et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): An update (2014–2018). Clin. Neurophysiol. 131, 474–528 (2020). [DOI] [PubMed] [Google Scholar]
- 99.Walton D, Spencer DC, Nevitt SJ & Michael BD Transcranial magnetic stimulation for the treatment of epilepsy. Cochrane Database Syst. Rev 2021, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nitsche MA & Paulus W Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol 527, 633 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sudbrack-Oliveira P et al. Transcranial direct current stimulation (tDCS) in the management of epilepsy: A systematic review. Seizure 86, 85–95 (2021). [DOI] [PubMed] [Google Scholar]
- 102.Kaye HL et al. Personalized, Multisession, Multichannel Transcranial Direct Current Stimulation in Medication-Refractory Focal Epilepsy: An Open-Label Study. J. Clin. Neurophysiol (2021). [DOI] [PubMed] [Google Scholar]
- 103.Daoud M et al. Stereo-EEG based personalized multichannel transcranial direct current stimulation in drug-resistant epilepsy. Clin. Neurophysiol 137, 142–151 (2022). [DOI] [PubMed] [Google Scholar]
- 104.Rezakhani S, Amiri M, Weckhuysen S & Keliris GA Therapeutic efficacy of seizure onset zone-targeting high-definition cathodal tDCS in patients with drug-resistant focal epilepsy. Clin. Neurophysiol 136, 219–227 (2022). [DOI] [PubMed] [Google Scholar]
- 105.Lefaucheur JP et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol 128, 56–92 (2017). [DOI] [PubMed] [Google Scholar]
- 106.Kravalis K & Schulze-Bonhage A PIMIDES I: a pilot study to assess the feasibility of patient-controlled neurostimulation with the EASEE® system to treat medically refractory focal epilepsy. Neurol. Res. Pract. 2020 21 2, 1–3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Filipescu C et al. The effect of medial pulvinar stimulation on temporal lobe seizures. Epilepsia 60, e25–e30 (2019). [DOI] [PubMed] [Google Scholar]
- 108.Kokkinos V et al. Responsive neurostimulation of the thalamus improves seizure control in idiopathic generalized epilepsy: A case report. Neurosurgery 87, E578–E583 (2020). [DOI] [PubMed] [Google Scholar]
- 109.Worrell GA Electrical Brain Stimulation for Epilepsy and Emerging Applications. J. Clin. Neurophysiol 38, 471–477 (2021). [DOI] [PubMed] [Google Scholar]
- 110.Alcala-Zermeno JL et al. Cortical and thalamic electrode implant followed by temporary continuous subthreshold stimulation yields long-term seizure freedom: A case report. Epilepsy Behav. Reports 14, 100390 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gregg NM et al. Thalamic deep brain stimulation modulates cycles of seizure risk in epilepsy. Sci. Reports 2021 111 11, 1–12 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morris GL & Mueller WM Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. Neurology 53, 1731–1735 (1999). [DOI] [PubMed] [Google Scholar]
- 113.Salinsky MC, Uthman BM, Ristanovic RK, Wernicke JF & Tarver WB Vagus nerve stimulation for the treatment of medically intractable seizures: Results of a 1-year open-extension trial. Arch. Neurol 53, 1176–1180 (1996). [DOI] [PubMed] [Google Scholar]
- 114.Sette AL et al. Battery longevity of neurostimulators in Parkinson disease: A historic cohort study. Brain Stimul. 12, 851–857 (2019). [DOI] [PubMed] [Google Scholar]