Abstract
Salmonella enterica serovar Pullorum is worldwide a poultry pathogen of considerable economic importance, particularly in those countries with a developing poultry industry. In addition to the characteristic high mortality rates among young chicks, one of the features of Salmonella serovar Pullorum infection is that it persists for long periods in convalescent chicks in the absence of clinical disease. This can lead to colonization of the reproductive tract of chickens and at sexual maturity can result in infected progeny through transovarian transmission to eggs. The sites of Salmonella serovar Pullorum persistence in convalescent birds are not known, and the mechanisms of persistence are not understood. Here we show that Salmonella serovar Pullorum can persist in both the spleen and the reproductive tract for over 40 weeks following experimental infection in chickens. During the period of sexual maturity, Salmonella serovar Pullorum colonized both the ovary and the oviduct of hens and led to 6% of laid eggs being infected by Salmonella serovar Pullorum. The colonization of several different sites of the reproductive tract suggests that Salmonella serovar Pullorum may employ more than one mechanism of egg infection. Persistence occurred despite a strong humoral response, suggesting an intracellular site of infection. By use of a Salmonella serovar Pullorum strain containing a plasmid stably expressing green fluorescent protein, we demonstrated that the main site of carriage in the spleen is within macrophages. This raises interesting questions about the biology of Salmonella serovar Pullorum, including why there is an increase in bacterial numbers when birds become sexually mature and in particular how Salmonella serovar Pullorum avoids clearance by macrophages and whether it modulates the immune system in other ways.
Salmonella enterica can be divided into two broad groups on the basis of pathogenesis and infection biology. One group consists of a large number of serovars, including Salmonella enterica serovar Typhimurium and Salmonella enterica serovar Enteritidis, that can colonize the alimentary tract of food animals or cause gastrointestinal disease in a range of hosts including humans. The other group comprises a small number of serovars that cause systemic typhoid-like disease in a restricted range of host species, such as Salmonella serovar Typhi in humans, Salmonella enterica serovar Dublin in cattle, and Salmonella enterica serovars Pullorum and Gallinarum in poultry. Several of these serotypes may, after clinical disease, persist in the tissues for long periods (6). Salmonella serovar Typhi and Salmonella serovar Dublin both localize in the gall bladder and spleen, and carriers may shed organisms for several years following infection (24, 41). Salmonella serovar Pullorum localizes in the reproductive tract of chickens and as a consequence may be transmitted vertically to chicks by transovarian transmission of the bacteria into developing hatching eggs (33). However, the mechanisms by which Salmonella serovar Pullorum and other serotypes persist within the host and the reasons for the absence of immune clearance are not known.
Salmonella serovar Pullorum is the causative agent of pullorum disease in poultry (33). Pullorum disease is an acute systemic disease that results in a high mortality rate in young chicks but rarely causes such severe clinical disease in adult birds, though it can result in loss of weight, decreased laying, diarrhea, and lesions and abnormalities of the reproductive tract (33). Control measures have virtually eradicated Salmonella serovar Pullorum from intensively reared commercial flocks in Europe and North America; there were just two reported isolations of Salmonella serovar Pullorum from poultry in the United Kingdom in 1998 (2). Both pullorum disease and fowl typhoid, caused by Salmonella serovar Gallinarum, have shown a tendency to return where free-range rearing of poultry has gained increasing popularity and continue to cause substantial economic losses of livestock in areas such as Asia and South America where the intensification of the poultry industry is in its infancy (27, 33). In these countries, high ambient temperatures and open-sided housing restrict the efficacy of hygiene measures because of environmental contamination. Wild and game birds may also act as a potential reservoir for Salmonella serovar Pullorum, which was isolated from game birds in the United Kingdom on 20 occasions in 1997 and 1998 (2). Pheasant chicks are also susceptible to pullorum disease (26). The importance of vertical transmission of infection between generations through eggs has long been recognized (32, 33, 36). Watanabe et al. (36) found high levels of maternal antibody to Salmonella serovar Pullorum in the eggs from some infected hens. These eggs were more likely to prevent multiplication of the bacteria within the eggs, leading to an increased chance of embryo survival and the hatching of an infected chick. Little is known regarding how Salmonella serovar Pullorum infects eggs or persists in the reproductive tract, other than that Salmonella serovar Pullorum appears to contaminate eggs in the ovary or oviduct following ovulation (33).
In this study, we aimed to investigate the long-term persistence of Salmonella serovar Pullorum in convalescent birds and vertical transmission to eggs, defining the sites of localization. We also determined specific antibody responses to Salmonella serovar Pullorum during a persistent infection. Using a Salmonella serovar Pullorum strain containing a plasmid expressing green fluorescent protein (GFP), we aimed to identify the main cell types associated with bacterial persistence in convalescent birds.
MATERIALS AND METHODS
Bacterial strains.
A spontaneous nalidixic acid-resistant mutant of Salmonella serovar Pullorum 449/87 (kindly provided by C. Wray, Veterinary Laboratory Agency, Weybridge, Surrey, United Kingdom) was grown from stocks maintained at −70°C in Luria-Bertani (LB) broth supplemented with 30% (vol/vol) glycerol. Bacteria were cultured for 18 h in LB broth at 37°C in an orbital shaking incubator at 150 rpm.
The Salmonella serovar Pullorum strain was transformed with the plasmid pBRD940 containing an ampicillin resistance marker and the gene for GFP under the control of the nirB promoter (kindly provided by L. Zhang Barber, University of Nottingham, Nottingham, United Kingdom). The use of the nirB promoter allows the stable expression of heterologous proteins within Salmonella under anaerobic conditions (8) and has been found previously to give high levels of expression of heterologous proteins by Salmonella within eukaryotic cells including macrophages (13).
Experimental animals.
Commercial brown-egg layers were obtained as 1-day-old chicks from a commercial poultry supplier. Birds were reared in wire cages at a temperature of 30°C, which was reduced to 20°C at 2 weeks of age. When birds came into lay at around 16 to 20 weeks of age, they were moved to individual laying cages, and eggs laid were collected daily. Birds were fed and watered ad libitum on a vegetable protein-based diet (SDS, Manea, Cambridgeshire, United Kingdom). Prior to the experiment, birds were checked for the absence of Salmonella infection by taking a cloacal swab, incubating it in selenite broth (Oxoid, Unipath, Basingstoke, Hampshire, United Kingdom) for 18 h, and then plating it onto Brilliant Green (BG) agar (Oxoid) containing 20 μg of sodium nalidixate/ml and 1 μg of novobiocin (Sigma Chemical Co., Poole, Dorset, United Kingdom)/ml (31).
Experiment 1: course of infection with Salmonella serovar Pullorum.
At 1 week of age, birds were infected orally with 109 organisms of Salmonella serovar Pullorum in an 0.2-ml volume of LB broth. Birds were killed at 1, 5, 10, 15, 20, 24, and 42 weeks postinfection, five birds being taken at each time point. Birds were bled prior to killing and at 1 day postinfection to obtain serum for determination of specific antibody response to Salmonella. Uninfected commercial layers were also raised similarly to act as controls and were bled at the same time as the infected birds. At postmortem examination, spleen, liver, bone marrow, breast muscle, heart tissue samples, and cecal contents were taken aseptically together with swabs of the cloaca and air sacs, and bacteriological analysis was performed as previously described (3, 32). Ovarian tissue and mature ovaries were removed aseptically, yolk from the developing follicles was collected in a jar containing selenite broth, and the remaining ovarian tissue was macerated with sterile scissors into a jar containing selenite broth. The samples were incubated at 37°C for 18 h and then plated onto selective BG agar containing 20 μg of sodium nalidixate/ml and 1 μg of novobiocin/ml. The oviduct was removed entire and cut lengthwise. The mucosal tissue was removed with a scalpel blade by scraping. Samples from the upper portion (infundibulum and magnum) and lower portion (isthmus and uterus) of the oviduct were removed separately. Samples were homogenized in a Griffiths tube and then plated onto selective BG agar as described above. Collected laid eggs and any developing eggs in the oviduct at postmortem were collected aseptically and placed into sterile glass jars, which were then shaken to break the egg and to mix the contents. The mixtures were incubated at 37°C for 18 h and then plated onto selective BG agar.
Experiment 2: determination of persistence in splenic macrophages.
As Salmonella serovar Pullorum was found to persist within the spleen for over 40 weeks in some birds following infection in experiment 1, it was decided to determine the main cell types involved in carriage and persistence despite a strong antibody response, suggesting an intracellular site. On the basis of the close interaction between Salmonella and the mononuclear phagocyte system (MPS) (reticuloendothelial system) in chickens (5), it was decided to specifically determine the rate of carriage within macrophages within the spleen.
Chickens were infected as described above with Salmonella serovar Pullorum 449/87(pBRD940), and birds were killed at 1 day, 3 days, 1 week, 5 weeks, and 10 weeks postinfection. At postmortem examination, spleens were taken as described above, and bacterial counts were determined on BG agar containing 20 μg of sodium nalidixate/ml and 50 μg of ampicillin/ml. Macrophages were isolated from spleen and liver on the basis of their adherent properties (10). Spleen and liver tissue samples were removed aseptically into Dulbecco's modified Eagle's medium (DMEM) and gently teased to form a cell homogenate. Cells were counted using a hemocytometer and adjusted to a concentration of 5 × 107/ml. This had been previously determined in a pilot experiment to be the optimum cell concentration to isolate macrophages from chicken spleen. Cells were seeded either in 24-well culture dishes (Nunc, Roskilde, Denmark) for determination of numbers of intracellular bacteria or onto coverslips for visualization of the intracellular bacteria. Cells were then incubated at 37°C in 5% CO2 for 4 h to allow the adherence of macrophages. After incubation, cells were washed vigorously four times with DMEM to remove nonadherent cells. The remaining adherent cells were incubated for 1 h in DMEM containing 100 μg of gentamicin sulfate (Sigma Chemical Co.)/ml to kill any extracellular bacteria. Immunofluorescent staining with the mouse anti-chicken macrophage monoclonal antibody KUL01 (20) was carried out to determine the percentage of macrophages in the adherent cell population. Macrophages were found to be in excess of 95% of the cell population, with the majority of contaminating cells being thrombocytes, adherent nucleated cells that are the equivalent of platelets in the chicken (38). After 1 h of incubation with medium containing gentamicin, cells were washed three times with DMEM. To determine the intracellular numbers of bacteria, macrophages were lysed with 1% (vol/vol) Triton X-100 in phosphate-buffered saline (Sigma Chemical Co.) for 20 min, and the lysate was plated on selective BG agar. To visualize intracellular bacteria expressing GFP, the cells were counterstained with wheat germ agglutinin conjugated with Texas Red-X (Molecular Probes, Eugene, Oreg.) and then observed either by standard fluorescence microscopy or by confocal laser microscopy.
Anti-Salmonella IgG response.
The serum immunoglobulin G (IgG) antibody response to Salmonella serovar Pullorum was determined by enzyme-linked immunosorbent assay (ELISA) using sonicated killed Salmonella serovar Pullorum as coating antigen as described by Barrow et al. (4).
RESULTS
Experiment 1: pathobiology and persistence of Salmonella serovar Pullorum in chickens and transmission to eggs.
At postmortem, lesions characteristic of Salmonella serovar Pullorum were found in the spleen, liver, and heart in two birds at 1 week postinfection and in all birds at 5 weeks postinfection, indicative of the acute systemic infection in the early stages of the experiment. This was accompanied by pronounced splenomegaly at 5 weeks postinfection. The number and severity of lesions decreased with time, indicating convalescence. At 20 weeks postinfection onwards, abnormalities and lesions of the ovaries and follicles were found in individual birds, with two birds also displaying signs of pericarditis at this time. Throughout the course of the experiment, 1 bird died out of 42. The bird died at 40 weeks postinfection, and at postmortem, this bird showed abnormality of the ovary and the spleen and liver cultured positive for Salmonella serovar Pullorum.
At 1 week following infection, Salmonella serovar Pullorum was recovered from all tissues in the majority of birds (Table 1), indicating a widespread systemic infection. However, by 5 weeks postinfection bacteria were no longer recovered from breast muscle, cecal contents, and bone marrow or the numbers recovered were vastly reduced. Relatively high numbers could still be recovered from the spleen and heart. Cloacal swabs were negative for the presence of Salmonella serovar Pullorum, but the bacteria were detected in air sacs of two of five birds. By 10 weeks postinfection, Salmonella serovar Pullorum was eliminated from most tissues and from the surface of the air sacs and cloaca but could still be recovered from the spleen and reproductive tract of individual birds up to 42 weeks following infection. It was noticeable that the numbers of Salmonella bacteria recovered in the spleen and, to a lesser extent, the reproductive tract and liver decreased from 10 to 15 weeks postinfection but increased as the birds came into lay at 20 weeks postinfection onwards. This was not the same for the heart, where the viable bacterial numbers continued to decrease. These data suggest that the spleen, reproductive tract, and, to a lesser extent, the liver and heart are sites of carriage of Salmonella serovar Pullorum in the chicken.
TABLE 1.
Mean viable counts of Salmonella serovar Pullorum 449/87 recovered from organs and tissues from experimentally infected commercial laying hens 1 to 42 weeks following infection (± standard errors of the means)a
| Tissue | Time (wk) postinfection
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1
|
5
|
10
|
15
|
20
|
24
|
42
|
||||||||
| No. positive/ no. total | Mean count (± SEM) | No positive/ no. total | Mean count (± SEM) | No. positive/ no. total | Mean count (± SEM) | No. positive/ no. total | Mean count (± SEM) | No. positive/ no. total | Mean count (± SEM) | No. positive no. total | Mean count (± SEM) | No. positive/ no. total | Mean count (± SEM) | |
| Spleen | 5/5 | 4.7 × 104 (±2.2) | 5/5 | 3.2 × 104 (±2.5) | 1/5 | 1.6 × 102 (±1.6) | 1/5 | + | 2/5 | 2.5 × 102 (±2.4) | 1/5 | 4.8 × 103 (±4.8) | 5/10 | 2.5 × 102 (±1.6) |
| Liver | 5/5 | 2.0 × 105 (±0.9) | 5/5 | 9.2 × 102 (±6.5) | 0/5 | − | 1/5 | + | 1/5 | + | 1/5 | 80 (±80) | 3/10 | 60 (±43) |
| Ovarian tissue | 5/5 | 1.5 × 104 (±0.6) | 1/5 | + | 1/5 | + | 1/5 | + | 1/3 | + | 3/5 | + | 5/10 | + |
| Yolk in ovary | ND | ND | ND | ND | ND | ND | ND | ND | 1/3 | + | 3/5 | + | 4/10 | + |
| Upper oviduct | ND | ND | ND | ND | ND | ND | ND | ND | 1/3 | 6.0 × 102 (±6.0) | 2/5 | 82 (±80) | 3/10 | 20 (±20) |
| Lower oviduct | ND | ND | ND | ND | ND | ND | ND | ND | 1/3 | 6.0 × 103 (±6.0) | 2/5 | 6.0 × 102 (±4.1) | 4/10 | 1.5 × 102 (±1.1) |
| Eggs in oviduct | ND | ND | ND | ND | ND | ND | ND | ND | 1/3 | ND | 2/3 | ND | 1/8 | ND |
| Heart | 5/5 | 7.6 × 104 (±4.1) | 1/5 | 3.2 × 103 (±3.2) | 2/5 | 2.8 × 102 (±1.7) | 1/5 | 3.2 × 103 (±3.2) | 2/5 | 20 (±20) | 0/5 | − | 2/10 | 33 (±33) |
| Cecal contents | 4/5 | 6.8 × 103 (±4.2) | 0/5 | − | 0/5 | − | 0/5 | − | 0/5 | − | 0/5 | − | 1/10 | 5.6 × 103 (±5.6) |
| Breast muscle | 2/5 | + | 0/5 | − | 0/5 | − | 0/5 | − | 0/5 | − | 0/5 | − | 0/5 | − |
| Bone marrow | 4/5 | 4.4 × 103 (±1.8) | 2/5 | 80 (±80) | 0/5 | − | 0/5 | − | 0/5 | − | 0/5 | − | ND | ND |
Five birds were taken for postmortem analysis at each time point, except at 42 weeks postinfection, when 10 were taken. ND, not done; +, positive culture by enrichment; −, negative culture following enrichment.
A total of 1,243 laid eggs were collected throughout the course of the experiment, 81 of which cultured positive for Salmonella serovar Pullorum (6.5%). Developing follicles in the ovary and developing eggs in the oviduct also cultured positive for Salmonella serovar Pullorum (Table 1), indicating that vertical transmission had occurred through infection of the developing egg, rather than contamination during or after lay. Of the 10 birds remaining at 42 weeks postinfection, 4 laid no positive eggs throughout the course of the experiment. No Salmonella serovar Pullorum bacteria were recovered from the tissues of these birds at postmortem. Of the six birds that laid eggs that cultured positive for Salmonella serovar Pullorum, Salmonella bacteria were recovered from the spleen and ovary of five birds and from the oviduct of four birds.
Anti-Salmonella IgG.
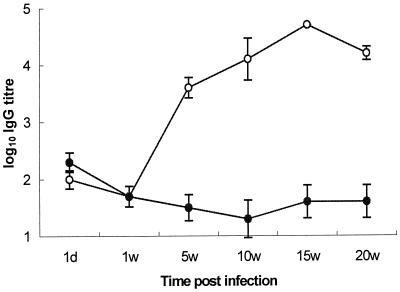
High titers of anti-Salmonella IgG were produced by infected birds from 5 weeks postinfection onwards (Fig. 1). Both infected and control birds showed indications of low levels of specific antibody at the time of infection, but this decreased at 1 week postinfection and further by 5 weeks in the controls.
FIG. 1.
Mean anti-Salmonella serovar Pullorum IgG titers in Salmonella serovar Pullorum-infected (n = 5) and uninfected (n = 3) commercial laying hens at postmortem (± standard errors of the means). Antibody titers were determined by ELISA using killed whole Salmonella serovar Pullorum sonicate as antigen. ○, IgG titer in Salmonella serovar Pullorum-infected birds; ●, IgG titer in uninfected controls. d, day; w, week.
Experiment 2: detection and persistence of Salmonella in macrophages.
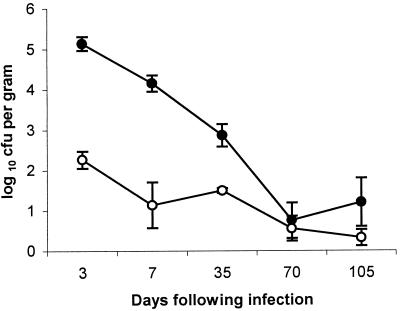
Salmonella serovar Pullorum was detected in splenic macrophages from 3 days postinfection to 10 weeks postinfection (Fig. 2). At 3 days to 5 weeks postinfection, Salmonella serovar Pullorum was recovered from the spleens and splenic macrophages of all infected birds examined. At 10 weeks postinfection, Salmonella serovar Pullorum was recovered from spleens and macrophages of three out of five birds. In the early stages of infection, the numbers of bacteria within macrophages formed only a small part of the total viable count of Salmonella bacteria, though by 5 and 10 weeks postinfection intracellular bacteria formed a substantial part of the total count. This correlates with the previous data (Table 1) that indicate that in the initial stages of infection high numbers of Salmonella serovar Pullorum bacteria are found at sites throughout the bird but are cleared by 5 weeks postinfection. The bacteria then persist in a limited number of sites such as the spleen, where they persist in low numbers despite a strong antibody response. This suggests that bacteria are found at a number of sites in addition to an intracellular macrophage location early in infection but are cleared by the immune system but also that the majority of persistent Salmonella serovar Pullorum bacteria are located primarily within macrophages despite the presence of high titers of specific IgG.
FIG. 2.
Mean viable counts (± standard errors of the means) of Salmonella serovar Pullorum 449/87(pBRD940) bacteria per gram of tissue in spleen and splenic macrophages following experimental infection of commercial laying hens. At each time point, five birds were taken for postmortem analysis. ●, total viable counts of Salmonella serovar Pullorum bacteria per gram of splenic tissue; ○, viable counts of intracellular Salmonella serovar Pullorum bacteria in splenic macrophages.
Salmonella serovar Pullorum 449/87(pBRD940) stably expressed GFP in vivo up to 10 weeks postinfection, allowing the visualization of intracellular bacteria within the macrophages (Fig. 3). At 1 week postinfection, approximately 1 to 2% of macrophages contained fluorescent Salmonella bacteria in all birds examined, with up to four bacteria per infected cell. This dropped to less than 1% at 5 weeks postinfection and even further at 10 weeks to less than 0.5% of cells infected. The numbers of bacteria per cell also fell, with only one or two bacteria per cell found. This decrease in fluorescent bacteria seen by microscopy correlates with the fall in bacterial counts in the spleen and splenic macrophages. Salmonella bacteria expressing GFP were found visually in all birds positive by bacteriology with the exception of one bird at 5 weeks postinfection. This indicates that visualization of Salmonella expressing GFP can be used reliably to detect bacteria in vivo, even when, as is the case here, small numbers are involved.
FIG. 3.
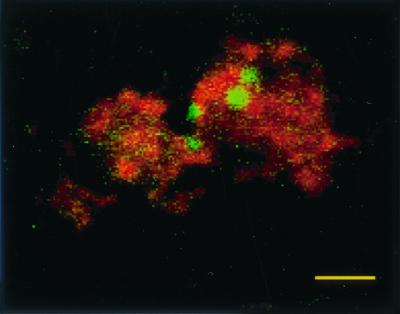
Confocal laser micrograph of Salmonella serovar Pullorum 449/87(pBRD940) expressing GFP within splenic macrophages counterstained with wheat germ agglutinin–Texas Red-X. Macrophages were isolated from infected birds 21 days following infection. Bar = 10 μm.
DISCUSSION
We have shown that, following infection of young birds, which resulted in clinical disease, convalescence was characterized by persistence of Salmonella serovar Pullorum in very low numbers in the MPS, reproductive tract, and heart. The latter location may be associated with the lesions in the myocardium which characterize chronic cases of infection. It was interesting that, unlike the bacterial numbers in the heart, which continued to decline throughout the experiment, those in the spleen and reproductive tract increased as the birds reached sexual maturity. Although the isolation of Salmonella serovar Pullorum from the reproductive tract of poultry has been reported many times (32, 33, 36), this is, we believe, the first record of persistent infection in the MPS.
Given the association between Salmonella and intracellular survival in macrophages which can be regarded as safe sites for bacterial multiplication (1, 12), we sought to determine whether Salmonella serovar Pullorum persisted within avian macrophages in vivo. The separation of splenic macrophages on the basis of their adherence allowed us to demonstrate that Salmonella serovar Pullorum persists within splenic macrophages, and after the initial acute stage of the infection, where high levels of extracellular bacteria were found, these form the majority of the organisms persisting within the spleen. This was demonstrated by both quantitative bacteriology and fluorescence. Persistence occurred despite high levels of circulating specific antibody against Salmonella serovar Pullorum. The intracellular location of the bacteria means that, although an effective antibody response is generated and is likely to be important in clearing extracellular bacteria, the intracellular bacteria survive. Low levels of specific antibody were found in control birds at the start of the experiment. This is likely to be maternal antibody, as commercial breeder birds and laying hens in the United Kingdom are now routinely vaccinated with a commercial killed Salmonella vaccine (11). Significant levels of specific IgG to Salmonella have previously been shown in the serum of the progeny of vaccinated breeder birds (21).
How Salmonella serovar Pullorum survives within macrophages is unclear. It seems likely that the type III secretion system encoded by Salmonella pathogenicity island 2 (SPI 2) may play a major role. SPI 2 is required for both survival in macrophages and virulence of Salmonella serovar Typhimurium in the murine infection model (9, 14). One of the functions of SPI 2 is to inhibit NADPH oxidase-dependent killing of Salmonella (35). Macrophage killing of Salmonella by respiratory burst has been shown to be one of the main mechanisms by which chickens genetically resistant to systemic salmonellosis clear the infection (P. Wigley, S. Hulme, N. Bumstead, and P. Barrow, unpublished results). Therefore, it seems likely that inhibition of macrophage oxidase activity would be vital if persistence within macrophages is to occur. Salmonella serovar Pullorum may also modulate the immune system in other ways. The high antibody titer indicates that a strong humoral response is driven by Salmonella serovar Pullorum infection, but the role of cellular responses in infection is not known. Down-regulation of cellular responses could inhibit macrophage activation, preventing clearance of intracellular Salmonella. Measuring cytokine responses in the spleen may give an indication of the main cell types involved in Salmonella serovar Pullorum infection and their regulation. Determination of cytokine responses in chickens has been difficult due to lack of available antibodies or suitable bioassays. The recent development of quantitative reverse transcription-PCR methods to determine the levels of a number of cytokines will allow a greater understanding of immune responses in the chicken to bacterial infection (17). The fate of macrophages infected by Salmonella serovar Pullorum is also not known. It is possible that bacteria persist within macrophages for the life span of the macrophage, which is up to 70 days in mice (28), and then infect a new macrophage or are cleared at cell death. This would explain the gradual decline in both intramacrophage and total bacteria in the spleen up to 10 weeks postinfection. In the mouse infection model, Salmonella serovar Typhimurium purA and aroA attenuated mutants have been shown to persist in the spleen and liver, gradually decreasing to clearance at 10 weeks postinfection, but serovar Typhimurium aroA purA double mutants and purE mutants persist longer (22). This supports the suggestion that all the bacteria are not cleared at macrophage death. Alternatively, macrophages could be lysed by Salmonella-mediated mechanisms and the intracellular bacteria could infect new cells in a constant cycle of lysis and invasion, or Salmonella serovar Pullorum may induce macrophage apoptosis through the SPI 1 type III secretion system as serovar Typhimurium has been shown previously to act in mice (37). Apoptotic macrophages containing Salmonella may be phagocytosed by macrophages acting to clear apoptotic cells and then persist in these fresh cells in a cycle of apoptosis induction and phagocytosis. Alternatively, as Salmonella serovar Pullorum was found in the bone marrow in the early stages of infection, splenic macrophages at the end of their life span may be replaced by circulating monocytes originating from the bone marrow, where they have been infected during their development.
The mechanisms by which Salmonella serovar Pullorum colonizes the reproductive tract and is transmitted vertically are not known. Although colonization of the reproductive tract has been described previously (33), the data shown here indicate that bacteria persist in very low numbers prior to birds coming into lay. As the birds come into lay, numbers of bacteria isolated from the reproductive tract, spleen, and liver increase. This suggests that changes in production of gonadal steroid hormones associated with the bird reaching sexual maturity may play a role in this increase. A possible explanation of the changes in the reproductive tract is that the population of macrophages in both the ovary and the oviduct increases at sexual maturity in laying hens (7, 42). The increase in the ovary appears to be mediated by estrols such as diethylstilbestrol (7), while the increases in the oviduct, particularly in the stroma of the shell gland, are mediated by progesterones (42). It is possible that the influence of gonadal hormones may lead to an influx of persistently infected macrophages to the reproductive tract. The theory is supported by the higher rate and counts of Salmonella serovar Pullorum bacteria recovered from the lower oviduct (Table 1), since the highest levels of macrophages in young laying hens were found in the shell gland that is located in this region. Alternatively, the increase in bacterial numbers may be more simply due to alteration of immune function by the sex hormones. Relatively little is known regarding the effects of gonadal steroids on the chicken immune system. At high concentrations, gonadal steroids appear to have an immunosuppressive effect. At lower concentrations, the hormones appear to have a regulatory role, acting both to increase and to suppress immune activity in a variety of ways (19), though little is known of their effects on bacterial infections. However, the numbers of both lymphocytes and macrophages in the reproductive tract at sexual maturity (39) and the previously reported increases in numbers of these cells and in antibody production in the reproductive tract in experimental Salmonella serovar Enteritidis infection (40) suggest that chickens have a strong immune response in the reproductive tract. Therefore, a method of avoiding immune clearance, such as intracellular survival in macrophages, would be required by Salmonella serovar Pullorum to persist in the ovaries and oviduct. The colonization of the reproductive tract by Salmonella requires extensive further study to explain vertical transmission of Salmonella serovar Pullorum and the contamination of table eggs with Salmonella serovar Enteritidis. This is likely to prove difficult, as the numbers of colonizing bacteria are very low before the birds come into lay, and even following sexual maturity, the numbers are too low to easily use flow cytometry or immunocytochemical techniques. Even though the use of Salmonella serovar Pullorum bacteria expressing GFP was successful in determining the location within macrophages in the spleen, the low numbers of macrophages in the reproductive tract compared with those in the spleen made this technique of little use in detecting the sites of carriage in the oviduct. A possible alternative is the use of in situ PCR to determine the location of the bacteria, as the amplification steps should increase sensitivity of detection. Nevertheless, considerable technical hurdles will need to be overcome. The mechanisms by which Salmonella serovar Pullorum is transmitted to eggs are also still unclear. Recovery of Salmonella serovar Pullorum from the ovary and developing yolk suggests that transmission to the yolk in the ovary is the most likely route. However, recovery of Salmonella serovar Pullorum from the upper oviduct suggests that transmission to the developing albumen may also occur, and the recovery from the lower oviduct suggests that contamination through the shell gland and even trans-shell contamination may also occur. The lack of information as to which parts of the eggs are infected by Salmonella serovar Pullorum does not support or rule out any of these routes.
Disease-free long-term persistence by S. enterica serovars that typically produce typhoid infection in a host-restricted manner in immunologically mature, healthy adult animals has practical implications. In addition, the phenomenon is immunologically very interesting. Salmonella serovar Typhi is well known to localize in the gall bladder for up to several years, producing secondary cases by fecal contamination. However, no suitable animal models of this are available. Despite attempts to reproduce gall bladder localization in rabbits (34), there is no information as to whether this is the only or main site of localization. Salmonella serovar Dublin is known to persist in a variety of sites in convalescent calves, including the gall bladder and the spleen (41). In addition, localization in the udder may lead to long-term transmission in milk (23). Salmonella serovar Enteritidis produces typhoid-like disease in mice and systemic disease in young chickens, and there is considerable epidemiological and experimental evidence of egg transmission, which has consequences for human health through food-borne salmonellosis, in addition to problems associated with vertical transmission to progeny (3, 15, 18, 30). In this case, it has been suggested that Salmonella serovar Enteritidis attaches to and invades ovarian granulosa cells, which has been demonstrated in vitro, leading to subsequent infection of the membrane of the developing egg (29). However, other studies have indicated that yolk, albumen, and shells are all contaminated with Salmonella serovar Enteritidis, suggesting that other routes such as transoviductal transmission and fecal contamination may play an important role in egg infection (15, 16). However, unlike Salmonella serovar Pullorum, which may infect hatched eggs from birds infected at a very young age in which the bacteria persist in the tissues until sexual maturity, Salmonella serovar Enteritidis does not appear to persist in the tissues but colonizes the reproductive tract of older birds, leading to egg infection (15), probably through an ascending infection of the cloaca.
Despite the fact that persistent carriage leading to vertical transmission is known to be an important component of the infection cycle and epidemiology in Salmonella serovar Pullorum, nothing is known of the immunological parameters of this phenomenon. Because of the relative ease of working with Salmonella serovar Pullorum in poultry compared with Salmonella serovar Dublin in cattle or Salmonella serovar Typhi in humans, it represents a useful model system for studying disease-free persistence. This area of infection biology has been reviewed recently, and it was considered that initial investigation should attempt to define the localization of the bacteria and the cells involved and begin to attempt to determine why immunological clearance does not take place (6, 25). In this study, we have shown that Salmonella serovar Pullorum can persist in both the reproductive tract and the spleen of chickens for over 40 weeks following infection. Carrier birds can transmit the infection vertically through infected eggs. After an initial, mild, systemic disease, birds recover, showing little signs of clinical disease, and produce a strong antibody response. Salmonella serovar Pullorum evades the immune response by surviving intracellularly within macrophages. This is of interest both in terms of the biology of salmonellosis and to the poultry industry worldwide, where Salmonella serovar Pullorum remains an important pathogen and the vertical transmission of pullorum disease remains a serious economic threat to livestock. In addition to the use of Salmonella serovar Pullorum as a model of disease-free persistence, it also is a model to develop in further detail techniques suitable to studying the localization of Salmonella serovar Enteritidis in the reproductive tract and transmission to eggs.
ACKNOWLEDGMENTS
We thank Scott Hulme and Kaye Murphy for technical assistance, Drew Worth at the Edward Jenner Institute for Vaccine Research for help with the laser confocal microscopy, and T. F. Davison for his most helpful advice.
We thank MAFF and BBSRC for support.
REFERENCES
- 1.Abshire K Z, Neidhardt F C. Analysis of proteins synthesized by Salmonella typhimurium during growth within a host macrophage. J Bacteriol. 1993;175:3734–3743. doi: 10.1128/jb.175.12.3734-3743.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. Salmonella in livestock 1998. London, United Kingdom: Veterinary Laboratory Agency/Ministry of Agriculture, Fisheries and Foods; 1999. [Google Scholar]
- 3.Barrow P A, Lovell M A. Experimental infection of egg-laying hens with Salmonella enteritidis phage type 4. Avian Pathol. 1991;42:194–199. doi: 10.1080/03079459108418769. [DOI] [PubMed] [Google Scholar]
- 4.Barrow P A, Berchieri A, Jr, Al-Haddad O. The serological response of chickens infected with Salmonella gallinarum-pullorum detected by ELISA. Avian Dis. 1992;36:227–236. [PubMed] [Google Scholar]
- 5.Barrow P A, Huggins M B, Lovell M A. Host specificity of Salmonella infections in chickens and mice is expressed in vivo primarily at the level of the reticuloendothelial system. Infect Immun. 1994;62:4602–4610. doi: 10.1128/iai.62.10.4602-4610.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrow P A, Duchet-Suchaux M. Salmonella and Salmonellosis '97 proceedings. Ploufragan, France: Zoopôle; 1997. Salmonella carriage and the carrier state; pp. 241–250. [Google Scholar]
- 7.Barua A, Yoshimura Y, Tamura T. The effects of age and sex steroids on the macrophage population in the ovary of the chicken, Gallus domesticus. J Reprod Fertil. 1998;114:253–258. doi: 10.1530/jrf.0.1140253. [DOI] [PubMed] [Google Scholar]
- 8.Chatfield S N, Charles I G, Makoff A J, Oxer M D, Dougan G, Pickard D, Slater D, Fairweather N F. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose tetanus vaccine. Bio/Technology. 1992;10:888–892. doi: 10.1038/nbt0892-888. [DOI] [PubMed] [Google Scholar]
- 9.Cirillo D M, Valdivia R H, Monack D M, Falkow S. Macrophage-dependent induction of the Salmonella pathogenicity island 2 type III secretion system and its role in intracellular survival. Mol Microbiol. 1998;30:175–188. doi: 10.1046/j.1365-2958.1998.01048.x. [DOI] [PubMed] [Google Scholar]
- 10.Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W. Current protocols in immunology. Vol. 2. New York, N.Y: John Wiley & Sons, Inc.; 1997. [Google Scholar]
- 11.Cruickshank G. Poultry world welfare guide no. 1: commercial layers. Sutton, Surrey, United Kingdom: Reed Business Information; 2000. [Google Scholar]
- 12.Dunlap N E, Benjamin W N, McCall R D, Tilden A B, Briles D E. A safe site for Salmonella typhimurium is within splenic cells during the early phase of infection within mice. Microb Pathog. 1991;10:297–310. doi: 10.1016/0882-4010(91)90013-z. [DOI] [PubMed] [Google Scholar]
- 13.Everest P, Frankel G, Li J, Lund P, Chatfield S, Dougan G. Expression of LacZ from the htrA, nirB and groE promoters in a Salmonella vaccine strain: influence of growth in mammalian cells. FEMS Microbiol Lett. 1995;126:97–101. doi: 10.1111/j.1574-6968.1995.tb07398.x. [DOI] [PubMed] [Google Scholar]
- 14.Hensel M, Shea J E, Waterman S R, Mundy R, Nikolaus T, Banks G, Vasquez-Torres A, Gleeson C, Fang F C, Holden D W. Genes encoding putative effector proteins of the type III secretion system of Salmonella pathogenicity island 2 are required for bacterial virulence and proliferation in macrophages. Mol Microbiol. 1998;30:163–174. doi: 10.1046/j.1365-2958.1998.01047.x. [DOI] [PubMed] [Google Scholar]
- 15.Humphrey T J. Contamination of eggs with potential human pathogens. In: Board R G, Fuller R, editors. Microbiology of the avian egg. London, United Kingdom: Chapman and Hall; 1994. pp. 93–116. [Google Scholar]
- 16.Humphrey T J. Contamination of eggs and poultry meat with Salmonella enterica serovar Enteritidis. In: Saeed A M, Gast R K, Wall P G, editors. Salmonella enterica serovar Enteritidis in humans and animals: epidemiology, pathogenesis and control. Ames: Iowa State University Press; 1999. pp. 183–192. [Google Scholar]
- 17.Kaiser P, Rothwell L, Galyov E E, Barrow P A, Burnside J, Wigley P. Differential cytokine expression in avian cells in response to invasion by Salmonella typhimurium, S. enteritidis and S. gallinarum. Microbiology. 2000;146:3217–3226. doi: 10.1099/00221287-146-12-3217. [DOI] [PubMed] [Google Scholar]
- 18.Keller L H, Benson C E, Krotec K, Eckroade R J. Salmonella enteritidis colonization of the reproductive tract and forming and freshly laid eggs of chickens. Infect Immun. 1995;63:2443–2449. doi: 10.1128/iai.63.7.2443-2449.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marsh J A, Scanes C G. Neuroendocrine-immune interactions. Poult Sci. 1994;73:1049–1061. doi: 10.3382/ps.0731049. [DOI] [PubMed] [Google Scholar]
- 20.Mast J, Gooderis B M, Peeters K, Vandesande F, Berghman L R. Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody KUL01. Vet Immunol Immunopathol. 1998;61:343–357. doi: 10.1016/s0165-2427(97)00152-9. [DOI] [PubMed] [Google Scholar]
- 21.Methner U, Steinbach G. Efficacy of maternal Salmonella antibodies and experimental oral infection of chicks with Salmonella enteritidis. Berl Muench Tieraerztl Wochenschr. 1997;110:373–377. [PubMed] [Google Scholar]
- 22.O'Callaghan D, Maskell D, Liew F Y, Easmon C, Dougan G. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attenuation, persistence, and ability to produce immunity in BALB/c mice. Infect Immun. 1988;56:419–423. doi: 10.1128/iai.56.2.419-423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Osborne A D, Pearson H, Linton A H, Shimeld C M. Epidemiology of Salmonella infection in calves: the source of calfhood infection with Salmonella dublin. Vet Rec. 1977;101:513–516. [PubMed] [Google Scholar]
- 24.Parker M T. Enteric infections; typhoid and paratyphoid fever. In: Parker M T, Collier L H, editors. Topley & Wilson's principles of bacteriology, virology and immunity. 8th ed. 3. Bacterial diseases. London, United Kingdom: Edward Arnold; 1990. pp. 423–446. [Google Scholar]
- 25.Penn C W. Chronic infections: latency and the carrier state. In: Hormaeche C E, Penn C W, Smyth C J, editors. Molecular biology of the bacterial infection. Forty-Ninth Symposium of the Society for General Microbiology. Cambridge, United Kingdom: Cambridge University Press; 1992. pp. 107–125. [Google Scholar]
- 26.Pennycott T W, Duncan G. Salmonella pullorum in the common pheasant (Phaisanus colchicus) Vet Rec. 1999;144:283–287. doi: 10.1136/vr.144.11.283. [DOI] [PubMed] [Google Scholar]
- 27.Pomeroy B S, Nagaraja K V. Fowl typhoid. In: Calnek B W, editor. Diseases of poultry. 9th ed. Ames: Iowa State University Press; 1991. pp. 87–98. [Google Scholar]
- 28.Rayner D. Tissues and cells of the immune system. In: Delves P J, editor. Cellular immunology labfax. Oxford, United Kingdom: Bios Scientific Publishers/Blackwell Scientific Publications; 1994. pp. 17–30. [Google Scholar]
- 29.Saeed A M, Thiaragarajan D, Asem E. Mechanism of transovarian transmission of Salmonella enterica serovar Enteritidis in laying hens. In: Saeed A M, Gast R K, Wall P G, editors. Salmonella enterica serovar Enteritidis in humans and animal: epidemiology, pathogenesis and control. Ames: Iowa State University Press; 1999. pp. 193–212. [Google Scholar]
- 30.Shivaprasad H L, Timoney J F, Morales S, Lucio B, Baker C. Pathogenesis of Salmonella enteritidis infection in laying chickens. I. Studies on egg transmission, clinical signs, fecal shedding, and serologic responses. Avian Dis. 1990;34:548–557. [PubMed] [Google Scholar]
- 31.Smith H W, Tucker J F. The effect of antibiotic therapy on the faecal excretion of Salmonella typhimurium by experimentally infected chickens. J Hyg. 1975;75:275–292. doi: 10.1017/s0022172400047306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snoyenbos G H, Smyser C F, Van Roekel H. Salmonella infections of the ovary and peritoneum of chickens. Avian Dis. 1969;34:668–670. [PubMed] [Google Scholar]
- 33.Snoyenbos G H. Pullorum disease. In: Calnek B W, editor. Diseases of poultry. 9th ed. Ames: Iowa State University Press; 1991. pp. 73–87. [Google Scholar]
- 34.Topley W W C, Wilson G S. Route of infection in typhoid fever. In: Topley W W C, Wilson G S, editors. The principles of bacteriology and immunity. 2nd ed. London, United Kingdom: Edward Arnold; 1936. pp. 1200–1201. [Google Scholar]
- 35.Vasquez-Torres A, Xu Y, Jones-Carson J, Holden D W, Lucia S M, Dinauer M C, Mastroeni P, Fang F C. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe S, Nagai T, Hashimoto K, Kume T, Sakazaki R. Studies on Salmonella infection in hens' eggs during incubation. VII. Transmission to eggs of agglutinins and immunity from hens infected with Salmonella serovar Pullorum. Bull Natl Inst Anim Health (Tokyo) 1960;39:37–41. [Google Scholar]
- 37.Weinrauch Y, Zychlinsky A. The induction of apoptosis by bacterial pathogens. Annu Rev Microbiol. 1999;53:155–187. doi: 10.1146/annurev.micro.53.1.155. [DOI] [PubMed] [Google Scholar]
- 38.Wigley P, Hulme S D, Barrow P A. Phagocytic and oxidative burst activity of chicken thrombocytes to Salmonella, Escherichia coli and other bacteria. Avian Pathol. 1999;28:567–572. doi: 10.1080/03079459994353. [DOI] [PubMed] [Google Scholar]
- 39.Withanage G S K, Baba E, Sasai K, Fukata T, Kuwamura M, Miyamoto T, Arawaka A. Localization and enumeration of T and B lymphocytes in the reproductive tract of laying hens. Poult Sci. 1997;76:671–676. doi: 10.1093/ps/76.5.671. [DOI] [PubMed] [Google Scholar]
- 40.Withanage G S K, Sasai K, Fukata T, Miyamoto T, Baba E, Lillehoj H S. T lymphocytes, B lymphocytes and macrophages in the ovaries and oviducts of laying hens experimentally infected with Salmonella enteritidis. Vet Immunol Immunopathol. 1998;66:173–184. doi: 10.1016/s0165-2427(98)00177-9. [DOI] [PubMed] [Google Scholar]
- 41.Wray C, Sojka W J. Reviews of the progress of dairy science: bovine salmonellosis. J Dairy Res. 1977;44:383–425. [PubMed] [Google Scholar]
- 42.Zheng W M, Yoshimura Y. Localization of macrophages in the chicken oviduct: effects of age and gonadal steroids. Poult Sci. 1999;78:1014–1018. doi: 10.1093/ps/78.7.1014. [DOI] [PubMed] [Google Scholar]