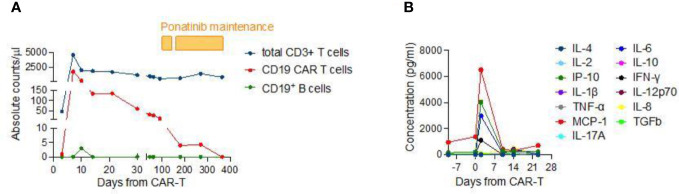
Figure 2.
(A) Longitudinal evaluation of total CD3+ T cells, anti-CD19 CAR-T cells, CD19+ B cells and released cytokines in the patient’s peripheral blood. Absolute counts were evaluated by flow cytometry at several time-points for up to 1 year after CAR-T cell infusion. The pharmacokinetics shows a remarkable CAR-T cell expansion in the first week following infusion (coinciding with CRS onset and resolution), with engineered cells representing nearly 40% of overall T cells, and a subsequent drop in CAR-T cells over time. At day 270, the patient still has a subset of CAR-T cells accounting for around 1% of the total T-cell count. At the time of last follow-up, circulating CAR-T cells had decreased below the detection limit of the assay but B-cell aplasia persists. (B) Evaluation of serum cytokines/chemokines concentrations in the first three weeks after CAR-T cell infusion. The analysis shows a significant peak occurring within the first week after treatment.