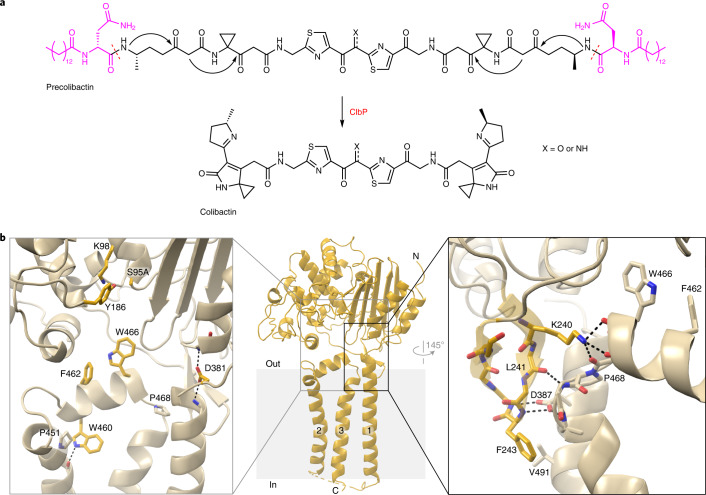
Fig. 1. The TMD of ClbP completes the substrate-binding site.
a, The proposed structure of colibactin is pseudodimeric and contains two electrophilic warheads that generate inter-strand cross-links in the DNA of epithelial cells in the human gut. To activate this toxin, the ClbP peptidase cleaves off the two prodrug motifs (colored in magenta) from the precursor molecule precolibactin, leading to non-enzymatic condensation to form the active warheads (curved arrows). b, The structure of full-length ClbP reveals an interface between the periplasmic and transmembrane domains. The inset on the left provides an expanded view of the interdomain interface. The conserved TMD residues and the catalytic triad are shown as sticks. The inset on the right shows interactions of the β3-β4 loop (dark yellow) with the TMD that likely stabilize the orientation of the catalytic site toward the cell membrane.