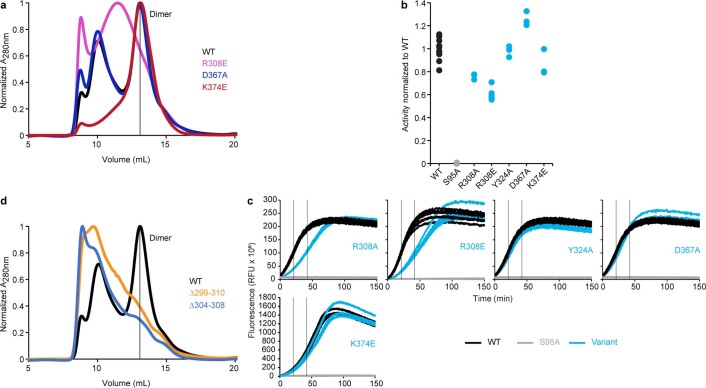
Extended Data Fig. 8. The dimer interface is important for the stability of ClbP.
a, Superposed normalized size exclusion chromatograms of wild-type ClbP and variants with mutations at the dimer interface performed on a Superdex 200 10/300 column. While no mutation yields a detectable monomeric species, R308E induces the formation of higher molecular weight aggregates. The vertical line indicates the elution volume of dimeric ClbP. b, Enzyme activity measurements of dimer interface variants normalized to the wild-type average, using the in vitro fluorogenic activity assay (number of experimental replicates: n = 13 (WT and S95A), 6 (R308E) or 3 (all others)). c, Raw fluorescence versus time curves for activity assays performed with dimer interface mutants. Each panel represents replicate measurements (n = 3 for R308A, Y324A, D367A, and K374E and n = 6 for R308E) for the indicated variant (cyan). For comparison, the corresponding replicate measurements for wild-type ClbP (black) and catalytically inactive S95A (gray), measured in the same experiment, are reproduced on each graph. The two gray vertical lines bound the data used for calculating the normalized hydrolysis rates in b. d, Superposed normalized size exclusion chromatograms of two constructs that replace residues 299-310 or 304-308, respectively, of the longest interface loop with a two-glycine linker. Both constructs elute primarily as higher molecular weight aggregates, suggesting the dimer interface is crucial for the integrity of biochemically isolated ClbP.