Fig. 3. N331 enforces d-asparagine specificity.
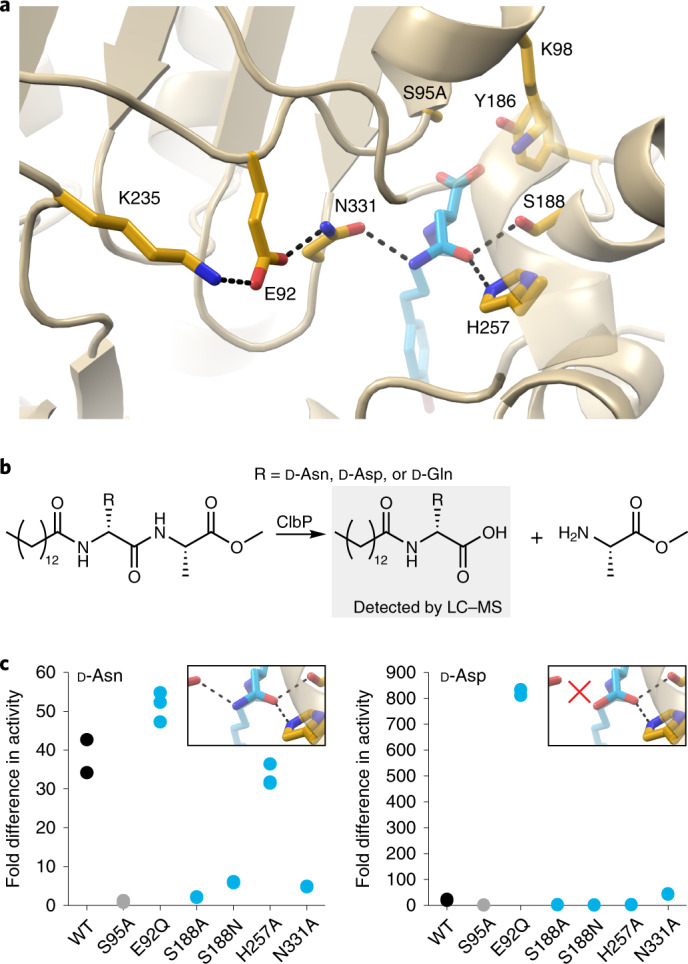
a, A network of interactions initiated by K235 orients N331 such that the carbonyl in its sidechain faces toward the binding pocket (the cartoon representation of residues 255–261 is transparent to optimize the view). b, Activity assay with substrate analogs containing prodrug motifs with alternative d-amino acids. Cleaved prodrug motif is detected by LC–MS (normalized to AUC of S95A) after a 5-hour incubation of the substrate with purified ClbP variants. c, Results of the assay in b for the substrate analogs containing d-Asn (left) or d-Asp (right); n = 3 independent experiments. None of the ClbP variants cleaved substantial amounts of the d-Gln-containing substrate analog (Extended Data Fig. 4d). Perturbing the orientation of the N331 sidechain allows ClbP to cleave d-aspartate substrates, suggesting that this residue is crucial for substrate specificity. Representative traces from the LC–MS are shown in Extended Data Fig. 4c. WT, wild-type.
