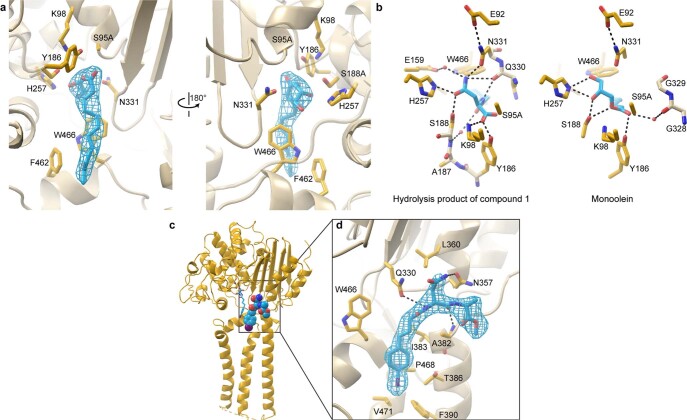
Extended Data Fig. 2. Comparison of the structures of ClbP in the presence of monoolein and a product analog illustrates that monoolein mimics a ClbP substrate or product.
a, Monoolein in the crystallization mesophase was trapped in the active site of one of our structures. The panels show two views, related by a 180° rotation, of a monoolein molecule (cyan) bound in the active site, with the corresponding electron density for a polder map contoured at 7σ. b, A side-by-side comparison of the active-site interactions of the (4-(4-bromophenyl)butanoyl)-d-asparagine product and monoolein illustrates that monoolein interacts similarly with active site residues that bind to the prodrug motif, explaining how it can outcompete substrate analogs introduced only in the precipitant solution but not the lipidic mesophase during the crystallization process. Hydrogen bonds are indicated as black dotted lines. c, In addition to the hydrolysis product in the active site (cyan sticks), we observed electron density corresponding to an intact substrate molecule at an adjacent site (cyan spheres). d, The inset shows sidechains within 4.2 Å of the bound substrate analog, with hydrogen bonds indicated as black dotted lines. The corresponding electron density for a polder map is contoured at 7σ.