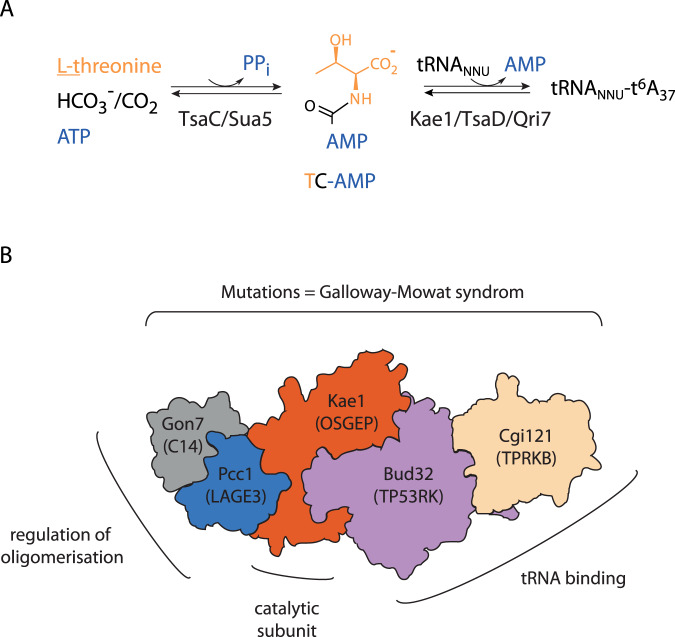
Fig. 1. KEOPS complex structure and function in t6A biosynthesis.
A Enzymatic synthesis of t6A. Sua5/TsaC protein family catalyzes the condensation of L-threonine, bicarbonate or CO2 and one ATP molecule to form an unstable intermediate threonylcarbamoyl-adenylate (TC-AMP). Kae1/TsaD/Qri7 family catalyzes the transfer of the threonylcarbamoyl moiety of TC-AMP to adenosine 37 within the anticodon loop to form t6A modified tRNA. B Schematic representation of the KEOPS structure and functional assignment of subunits. The complex is a linear assembly of five subunits Cgi121/Bud32/Kae1/Pcc1/Gon7. The names of the human orthologs are given in the brackets. Gon7/C14 subunit has been found only in fungi and animals. The shape of each subunit roughly recapitulates the corresponding crystal structures in Gon7-Pcc1 (PDB 4WXA), Pcc1-Kae1 (PDB 3ENO), Kae1-Bud32 (PDB 3EN9), and Bud32-Cgi121 (PDB 4WWA) binary complexes. Genetic and in vitro functional assays established that Bud32 and Cgi121 function in tRNA recognition and binding. Pcc1 forms homodimers and provides the interface for oligomerization of the four subunit (4SU) KEOPS complex into a dimer of two heterotetramers. In fungi and animals, the Gon7/C14 subunit binds to Pcc1 through its dimerization interface and blocks the assembly of the KEOPS dimer. Mutations in all five genes encoding human KEOPS were linked with a severe genetic disease called Galloway-Mowat syndrome.