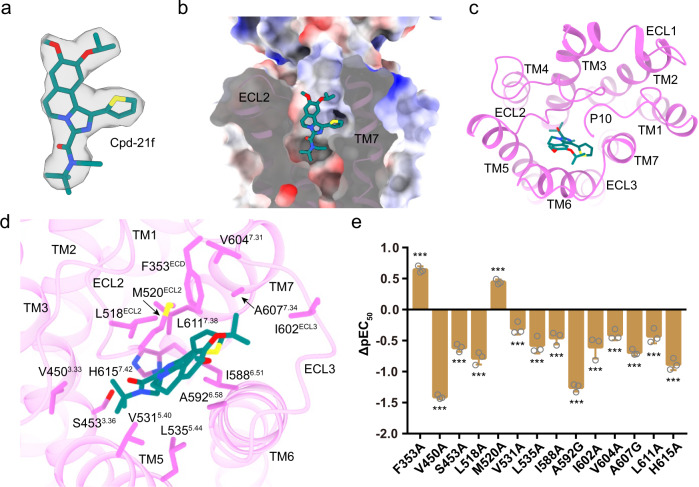
Fig. 4. Structural basis for FSHR activation by an allosteric agonist Cpd-21f.
a The structure and EM density of Cpd-21f in the bound structure. The density map is shown at level of 0.17. b, c The binding pocket of Cpd-21f in FSHR, from the front view (b) and top view (c). d Detailed interactions between Cpd-21f and FSHR. e Effects of different pocket mutations on the potency of Cpd-21f-induced cAMP accumulation. Data were shown as ΔpEC50 ± S.E.M. from three independent experiments, which performed in triplicates, with total repeats of nine for each data point. **P < 0.01, ***P < 0.001 versus WT. Statistical significance of differences between WT and mutants was determined by two-sided one-way ANOVA with Tukey test. Source data are provided as a Source Data file. All the exact P values are listed as follows: <0.0001, <0.0001, <0.0001, <0.0001, <0.0001, 0.0003, <0.0001, <0.0001, <0.0001, <0.0001, <0.0001, <0.0001, <0.0001, <0.0001.