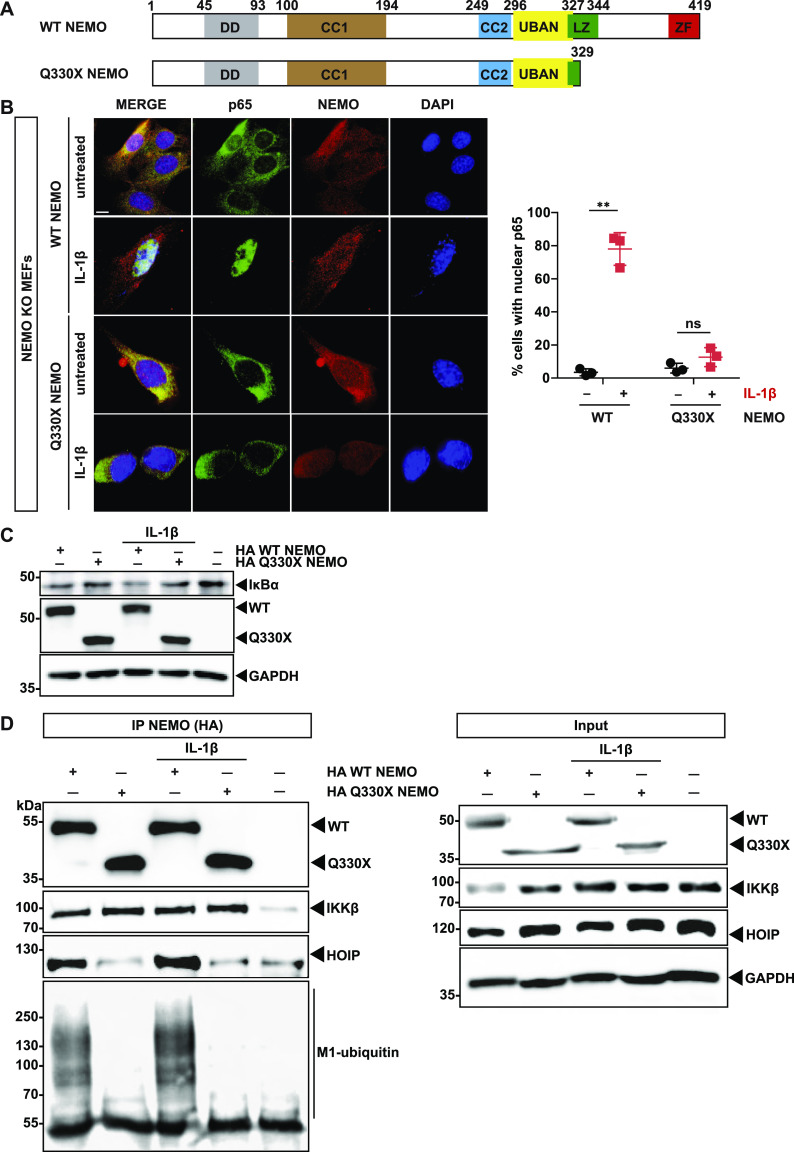
Figure 1. The pathogenic Q330X NEMO mutant is defective in NF-κB signaling.
(A) Domain structure of WT human NEMO and of the pathogenic mutant Q330X NEMO. DD, dimerization domain (aa 45–93); CC1, coiled-coil 1 domain (aa 100–194); CC2, coiled-coil 2 domain (aa 249–292); UBAN, ubiquitin binding in ABIN and NEMO (aa 296–327); LZ, leucine zipper (aa 322–344); ZF, zinc finger (aa 389–419). (B) Q330X NEMO does not promote p65 translocation upon IL-1β stimulation. NEMO KO MEFs were reconstituted with HA-tagged WT NEMO or Q330X NEMO. 24 h later, the cells were stimulated with murine IL-1β (20 ng/ml) for 15 min or left untreated and then analyzed by immunocytochemistry and SR-SIM using antibodies against NEMO and p65 (scale bar, 10 µm). Data are displayed as mean ± SD and were analyzed by Kruskal-Wallis test followed by Dunn’s Multiple Comparison Test, n = 3, **P ≤ 0.01. (C). WT NEMO but not Q330X NEMO induces IκBα degradation upon IL-1β stimulation. HEK293T cells were transiently transfected with HA-tagged WT NEMO or HA-Q330X NEMO, as indicated. 1 d after transfection, the cells were stimulated with human IL-1β (25 ng/ml) for 15 min or left untreated and analyzed by immunoblotting using antibodies against IκBα, NEMO, and GAPDH. (D) The Q330X mutation disrupts binding of NEMO to HOIP and M1-linked ubiquitin. HEK293T cells were transiently transfected with HA-tagged WT NEMO or Q330X NEMO, as indicated. 1 d after transfection, the cells were stimulated with human IL-1β (25 ng/ml) for 15 min or left untreated and then lysed. HA-tagged NEMO was immunoprecipitated using anti-HA-beads, followed by immunoblotting using antibodies against HA, IKKβ, HOIP, and M1-ubiquitin. The input was immunoblotted for NEMO, IKKβ, HOIP, and GAPDH.
Source data are available for this figure.