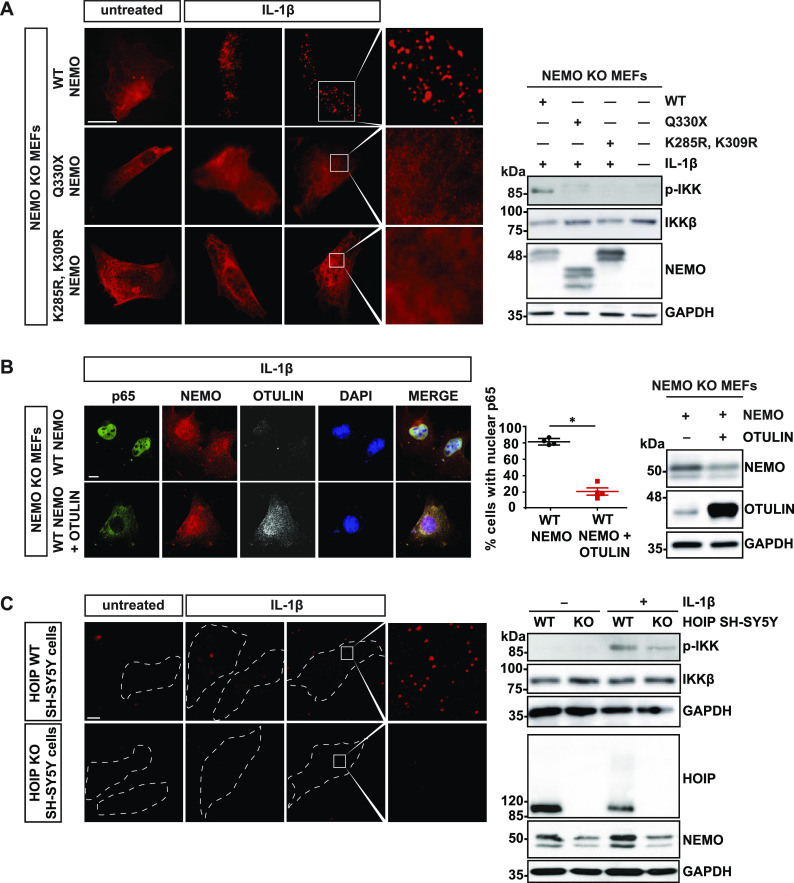
Figure 2. Linear ubiquitination promotes the formation of NEMO assemblies and IKK complex activation upon IL-1β receptor activation.
(A) In contrast to WT NEMO, Q330X and K285/309R NEMO do not form foci upon IL-1β treatment. NEMO KO MEFs were reconstituted with HA-tagged WT NEMO, Q330X NEMO or K285/309R NEMO. 24 h later, the cells were stimulated with murine IL-1β (20 ng/ml) for 15 min or left untreated and then analyzed after saponin extraction by immunocytochemistry using an antibody against NEMO (scale bar, 10 µm). Right Panel: IKK complex activation was analyzed by immunoblotting using an antibody against phospho-IKKα/β. Antibodies against IKKβ and NEMO were used to control expression levels. GAPDH was immunoblotted as input control. (B) IL-1β–induced nuclear translocation of p65 is impaired by OTULIN. The cells were treated as described in (A) and analyzed by immunocytochemistry using antibodies against NEMO, OTULIN, and p65 (scale bar, 10 µm). Right panel: Data are displayed as mean ± SD and were analyzed by Mann–Whitney test, n = 4, *P ≤ 0.05. Expression levels of HA-tagged WT NEMO and OTULIN were analyzed by immunoblotting using antibodies against NEMO and OTULIN. GAPDH was immunoblotted as input control. (C) IL-1β–induced formation of NEMO assemblies and IKK complex activation are compromised in HOIP-deficient cells. Control SH-SY5Y cells and HOIP KO SH-SY5Y cells were stimulated with human IL-1β (20 ng/ml) for 10 min and then analyzed by immunocytochemistry after saponin extraction using an antibody against NEMO (scale bar, 10 µm). Right Panel: IKK complex activation was analyzed by immunoblotting using an antibody against phospho-IKKα/β. Antibodies against IKKβ, HOIP, and NEMO were used to control expression. GAPDH was immunoblotted as input control.
Source data are available for this figure.